Administered activity and outcomes of glass versus resin 90Y microsphere radioembolization in patients with colorectal liver metastases
Introduction
Colorectal cancer is the third most common cancer worldwide, with nearly 1.4 million new cases diagnosed each year (1). Approximately 50–60% of patients with colorectal cancer present with or develop hepatic metastases through the course of their disease (2,3). Surgical liver resection offers the best prognosis for these patients but is only an option for 10–20% of patients diagnosed with colorectal liver metastases (2,4).
Over the past 2 decades, selective internal radiation therapy (SIRT) using yttrium-90 (90Y) microspheres (also called radioembolization) has emerged as an effective and safe treatment option for patients with unresectable metastatic colorectal cancer with liver-dominant metastases (5). 90Y microspheres emit beta radiation within a relatively small radius (mean 2.5 mm), delivering tumoricidal radiation doses to localized regions in the liver while limiting the dose to healthy liver tissue (6,7). Currently, 2 different 90Y microsphere products are commercially available: glass 90Y microspheres (TheraSphere®; Biocompatibles UK Ltd., Surrey, England) and resin 90Y microspheres (90Y-resin; SIR-Spheres®; SIRTeX Medical Limited, North Sydney, NSW, Australia) (6). Glass 90Y microspheres (90Y-glass) measure 20–30 µm in size and contain approximately 2,500 Bq per sphere, while resin 90Y microspheres (90Y-resin) measure 20–60 µm in size and contain 50 Bq per sphere. A compartmental model is the manufacturer-recommended method to calculate the appropriate activity of 90Y-glass, and the body surface area (BSA) method is recommended for the activity calculation of 90Y-resin (5,8).
Both 90Y-glass and 90Y-resin have shown efficacy in patients with unresectable liver metastases (9,10), however given their differences in specific activity, size, and dosimetry methods, these products may expose the liver to different amounts of radiation, thereby affecting their efficacy and tolerability. To date, no individual study has directly compared the dosimetry of 90Y-glass versus 90Y-resin used in SIRT in patients with unresectable hepatic metastases from colorectal cancer.
In this retrospective analysis of patients undergoing radioembolization for colorectal liver metastases at a single institution, we assessed the calculated prescribed radiation activity in patients treated with 90Y-glass versus 90Y-resin and compared efficacy and adverse event outcomes.
Methods
Patients
This study was a single-institution retrospective analysis of patients with unresectable colorectal liver metastases treated with 90Y microsphere radioembolization at Cleveland Clinic between June 11, 2008, and May 16, 2011.
Patient records, including contrast enhanced computed tomography (CT) or magnetic resonance (MR) images, were retrospectively reviewed. This study was approved by Cleveland Clinic’s Institutional Review Board and is compliant with the Health Insurance Portability and Accountability Act.
Radioembolization
Radioembolization was delivered via a hepatic artery catheter infusion with either 90Y-glass (TheraSphere®; Biocompatibles UK Ltd., Surrey, England) or 90Y-resin (SIR-Spheres®; SIRTeX Medical Limited, North Sydney, NSW, Australia) as previously described (5,11). The treatment strategy of either bilobar treatment, sequential lobar, or whole-liver 90Y radioembolization was at the discretion of the interventional radiologist. Selection of 90Y-glass or 90Y-resin was also determined by the managing interventional radiologist with discussion from the nuclear medicine authorized user, as needed.
90Y activity calculations
90Y activity was determined according to the manufacturer and recommended practice guidelines at the time of treatment (5,12-15).
For glass 90Y microspheres (TheraSphere®):
Target liver lobar volume was multiplied by 1.03 g/cm3 conversion factor to calculate target liver lobe mass. Target 90Y activity was determined by the following formula, using 120 Gy as the desired dose (5,15):
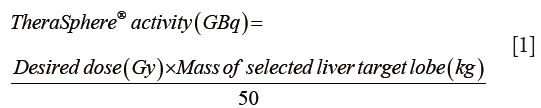
For resin 90Y microspheres (SIR-Spheres):
The target activity was calculated using the body surface area (BSA) method, adjusted for the fraction of the liver to be treated (5,12-14):
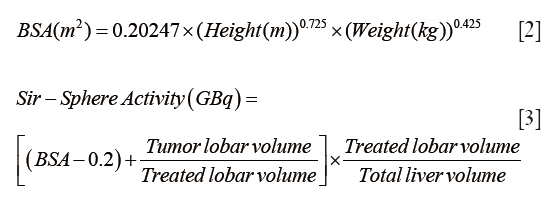
Prescribed 90Y activity was obtained from patient records. For each 90Y-glass treatment, the corresponding projected activity of 90Y-resin was calculated using the formula above, using tumor and liver volumes determined by MIM® software (Cleveland, OH, USA) analysis of baseline CT or MR images. For each 90Y-resin treatment, the corresponding projected activity of 90Y-glass was calculated using the formula above.
Evaluation of response and survival
CT or MR imaging was performed at baseline and at follow-up, typically 1 to 3 months posttreatment. Response assessment was performed according to Response Evaluation Criteria in Solid Tumors combined with necrosis criteria (mRECIST). Time to progression and overall survival time in months was calculated as the difference from the date of first 90Y radioembolization to date of the event or last observation date in case of censoring. The date of death was obtained from patient records. For patients lost to follow-up, date of death was obtained by searching available public records.
Toxicity assessments and adverse events
Laboratory data from tests performed at baseline and at follow-up observations were obtained from the patients’ charts, including liver function tests, hemoglobin, and blood cell counts. The presence and severity of adverse events occurring within 90 days of radioembolization and deemed to be related to the treatment were obtained from patient records, including fatigue, nausea/vomiting, abdominal pain, jaundice, cholecystitis, and gastritis. Liver enzyme toxicities were classified using the National Cancer Institute common terminology criteria for adverse events v4.03 (16).
Statistical analyses
Differences in baseline characteristics between the treatment groups were evaluated by a Wilcoxon rank sum test for ordinal variables and a Fisher’s exact test for categorical variables. Overall survival probabilities were evaluated using the Kaplan-Meier method with 95% confidence intervals (95% CI) for the difference in median survival time between 90Y-resin and 90Y-glass patients constructed with the percentile bootstrap method. Ten thousand bootstrap samples were generated, each sampled with replacement at the patient level. Fisher’s exact test was used to assess differences in the rate of disease progression (or death) between 90Y-resin and 90Y-glass patients at 3 and 6 months after first 90Y treatment. A Cox proportional hazards model was used to assess the effect of treatment on survival while adjusting for age and tumor extent at first treatment. In this model, treatment (90Y-glass vs. 90Y-resin) was included as the main predictor of interest, while age and tumor extent at first treatment were included as covariates. A significance level of 0.05 was used for all analyses.
Results
Patient characteristics and treatment strategy
Between June 11, 2008, and May 16, 2011, a total of 28 consecutive patients with colorectal liver metastases underwent transarterial radioembolization with 90Y microspheres at Cleveland Clinic. Fifteen patients received 29 treatments with 90Y-glass, and 13 patients received 22 treatments with 90Y-resin. All patients had received prior chemotherapy, typically multiple lines of chemotherapy. Baseline demographics and liver enzymes were similar between the 2 groups (Table 1). The median time from diagnosis of liver metastases to 90Y treatment was more than twice as long for 90Y-resin-treated patients vs. 90Y-glass–treated patients (448 vs. 184 days, respectively; P=0.025). The 90Y-glass prescribed activity per treatment was significantly higher than that of 90Y-resin [mean 1.77 gigabecquerel (GBq) vs. 1.05 GBq; P=0.007]. The mean administered activity per patient over the entire course of treatment was almost 3 times higher among 90Y-glass–treated patients compared to 90Y-resin–treated patients (mean: 3.5 vs. 1.3 GBq; P<0.001). Differences in lobar involvement and treatment plan also existed between the 90Y-resin and 90Y-glass groups. 90Y-resin–treated patients with bilobar involvement received treatment for each lobe on the same day, while 90Y-glass–treated patients typically received sequential lobar treatments ≤1 month apart.

Full table
90Y activity prescribed with glass vs. resin 90Y microspheres
By contouring tumors on baseline CT or MR images from patients in the 90Y-glass group, we calculated the 90Y-resin projected activity (Figure 1A,B) for each 90Y-glass radioembolization treatment. Conversely, by contouring the liver lobes on baseline CT or MR images from patients in the 90Y-resin group, we calculated the 90Y-glass projected activity (Figure 1C) for each 90Y-resin radioembolization treatment.
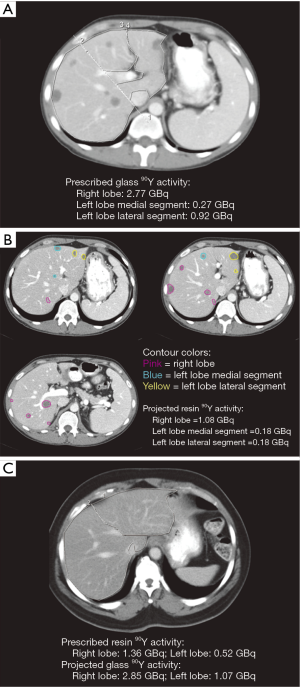
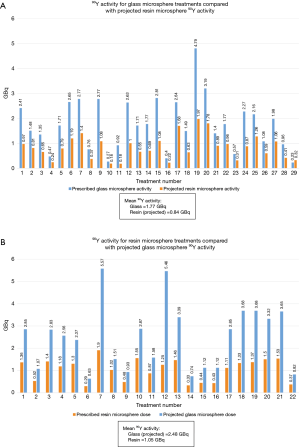
If patients in the 90Y-glass group had been treated with 90Y-resin, their mean radiation activity per treatment would have been 53% lower (mean prescribed 90Y-glass activity =1.77 GBq; mean projected 90Y-resin activity =0.84 GBq; Figure 2A). Likewise, if patients in the 90Y-resin group had been treated with 90Y-glass, their mean radiation activity per treatment would have been 136% higher (mean projected 90Y-glass activity =2.48 GBq; mean prescribed 90Y-resin activity =1.05 GBq; Figure 2B).
Time to progression and overall survival
Eleven of 14 patients (79%) treated with 90Y-glass and 8 of 13 patients (62%) treated with 90Y-resin progressed ≤3 months posttreatment (P=0.420). By 6 months, 79% of 90Y-glass–treated patients and 85% of 90Y-resin–treated patients showed progression (P=0.999).
The date of death was obtained for all 28 patients. Public records confirmed the date of death for 17 90Y microsphere patients lost to follow-up (8 90Y-glass, 9 90Y-resin). Median survival was 547 days (18.2 months)for 90Y-resin–treated patients as compared to 279 days(9.3 months) for 90Y-glass–treated patients, although the 268 day difference was not statistically significant (95% CI: −186–458; P=0.292; Figure 3). Of note, 2 90Y-glass patients and 1 90Y-resin patient had prolonged survival times (1,170 days, 1,225 days, and 1,259 days, respectively), and 2 90Y-resin patients died ≤40 days. Excluding the 3 outliers with prolonged survival from the analysis revealed a significantly longer overall survival for 90Y-resin–treated patients as compared with 90Y-glass–treated patients (496 vs. 273 days, respectively; P=0.021. No significant difference in survival between 90Y-glass–treated patients and 90Y-resin–treated patients was observed when 2 90Y-resin patients with survival ≤40 days were excluded from the analysis (279 vs. 603 days; P=0.121).
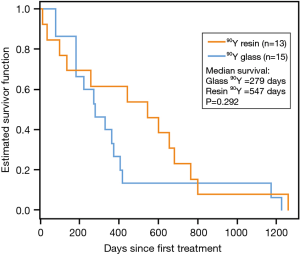
After adjusting for age and hepatic tumor burden, the hazard ratio (HR) of death for 90Y-glass–treated patients was an estimated 4 times greater than that of 90Y-resin–treated patients during the second year after initial SIRT (HR: 4.0; 95% CI: 1.3,12.3; P=0.017).
Total administered activity and survival
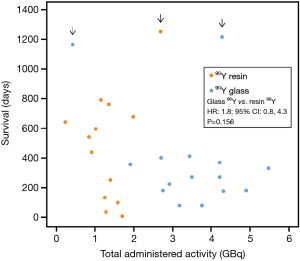
In all but 1 90Y-glass—treated patient, the total 90Y administered activity was higher in 90Y-glass—treated patients than in 90Y-resin–treated patients. After adjusting for age and hepatic tumor burden the observed survival tended to be higher for 90Y-resin–treated patients (Figure 4), although this difference was not statistically significant (HR 90Y-glass vs. 90Y-resin: 1.8; 95% CI: 0.8,4.3; P=0.156).
Clinical and laboratory toxicities
Fatigue was the most common reported symptom (90Y-glass group: 53%; 90Y-resin group: 77%; Tables 2,3). Most adverse events were mild and occurred within a month of the first 90Y treatment. Abdominal pain resolved without surgical intervention. No deaths were deemed to have resulted as a direct consequence of the treatments themselves. No patient experienced radioembolization-induced liver disease (REILD). Similar liver toxicities were observed in patients receiving 90Y-glass and 90Y-resin treatments.
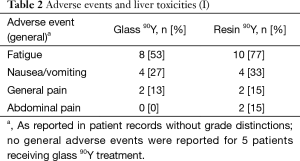
Full table
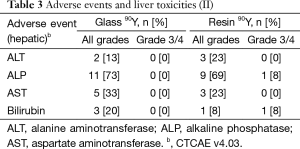
Full table
Conclusions
Using dosimetry calculations based on CT and MR images of real-world patients, we found that the treatment of a particular volume of liver tissue required significantly less 90Y activity with 90Y-resin when compared to 90Y-glass. Our results suggest a trend toward improved survival in patients receiving 90Y-resin, particularly during the second year following SIRT.
One of the core principles of radiation therapy is to keep radiation exposure as low as reasonably achievable (17). In our study, patients in the 90Y-glass group received almost 3 times the total liver radiation activity over the entire course of treatment than the 90Y-resin group received. When we compared the prescribed and corresponding projected activity for each 90Y treatment, we found that 90Y-glass treatment would more than double the 90Y activity that would be used if the same patient were treated with 90Y-resin. This significant finding raises the question of whether radiation dosage to normal liver tissue can be minimized by using 90Y-resin or by altering the dosimetry for 90Y-glass.
The median survival of 18.2 months (90Y-resin) and 9.3 months (90Y-glass) is in line with recently published studies of SIRT in patients with hepatic colorectal metastases (11,18-27). The results from these studies have shown a wide range for median survival of patients undergoing SIRT: 8.3–17.0 months with 90Y-resin (19,22-27) and 9.3–15.2 months with 90Y-glass (11,18,20,21). The extent of liver tumor burden, extrahepatic disease and number of prior chemotherapy lines have been shown to have a significant impact on overall survival following SIRT and may explain the variation in overall survival seen in our and prior studies (11,18,19,27,28). For the most part, our patients had ≤25% liver tumor burden with no recorded extrahepatic disease and were being treated in a salvage setting. The 3 90Y-resin patients with >25% tumor burden died at 15, 40, and 547 days; the 1 90Y-glass patient with >25% tumor burden died at 83 days. The longer interval between the diagnosis of liver metastases and SIRT in the 90Y-resin cohort might predict that this cohort would have a shorter overall survival compared to the 90Y-glass cohort, however we observed a nonsignificant trend toward longer survival in the 90Y-resin cohort. Due to the small size of this study and the significant difference in the interval between the diagnosis of metastases and SIRT, we cannot draw any strong conclusions when comparing overall survival with 90Y-resin and 90Y-glass.
During the second year following SIRT 90Y-glass—treated patients had roughly 4 times the hazard of death as compared to 90Y-resin—treated patients. One possible explanation for this finding could be that the lower 90Y activity of the 90Y-resin caused less insult to normal liver tissue than 90Y-glass. Recent 90Y PET/CT studies in 90Y-glass-treated patients have demonstrated an absorbed radiation dose of 67–94 Gy in normal parenchyma and found that complications increase with higher absorbed radiation doses to normal liver tissue (29,30). No patient in this small study experienced REILD, a rare but serious complication of SIRT that is associated with higher prescribed 90Y activity in patients treated for unresectable hepatic tumors (31,32). Although we observed no significant differences in adverse events or liver toxicities between the 90Y-glass or 90Y-resin groups, our findings were based on medical records that may not accurately capture all complications. Nevertheless, the authors would conjecture that the radiation exposure to normal liver parenchyma has enough biologic effect to influence overall survival, even though the exposures in both 90Y-glass and 90Y-resin groups do not typically reach the threshold required for inducing REILD.
A strength of this study is that these real-world patient cohorts were treated at the same institution and were similar in disease stage, prior chemotherapy, demographics, performance status, and liver function. Any cohort differences would not impact our finding that prescribed 90Y activity was significantly lower with 90Y-resin versus 90Y-glass per treatment, as we compared the prescribed activity and the projected activity to be used for the same treatment. We acknowledge that the interpretation of overall survival data is substantially limited by the small cohort size and retrospective design. Survival results may also have been impacted by differences in the 90Y-resin and 90Y-glass cohorts and concurrent chemotherapy in some patients. Our institution’s interventional radiologists’ particular practices may account for differences in the treatment strategies for the 90Y-glass and 90Y-resin groups, including the significantly longer interval between diagnosis of metastases and SIRT in the 90Y-resin group and the apparent preference for 90Y-resin for unilobar treatments and 90Y-glass for bilobar treatments. We also note that there was no uniform follow-up testing or systematic post-therapy imaging to confirm the distribution of 90Y, which has recently become more common in SIRT. At the Cleveland Clinic, post-therapy 90Y PET/CT has been the standard practice since 2012, but at the time of this cohort study Bremsstrahlung SPECT/CT scans were performed on only a few patients on an ad hoc basis.
In conclusion, our study shows that SIRT using 90Y-resin delivers significantly less 90Y activity to patients with liver-dominant colorectal metastases as compared to SIRT using 90Y-glass. Our results also suggest a possible survival benefit for patients treated with 90Y-resin as compared to those treated with 90Y-glass and a similar adverse event profile for both products. Larger, prospective studies are warranted to examine the effect of dosimetry on SIRT efficacy and safety outcomes.
Acknowledgements
The authors would like to thank Jennifer Nepo of Eubio Medical Communications for editorial support (funding provided by SIRTeX Medical Inc.). Special thanks to Drs. Sankaran Shrikanthan and Ram Gurajala at Cleveland Clinic for their helpful discussions over the course of the study.
Footnote
Conflicts of Interest: S.S. has served on advisory boards for Siemens and Bayer Healthcare and as a consultant for Siemens, has received speaker honoraria from SIRTeX Medical Inc. and BTG International, Inc., and has received payment for the development of educational presentations for Bayer Healthcare. V.K. received a speaker honorarium from SIRTeX Medical Inc. Authors E.N., J.B., and A.P. declare that they have no competing interests. S.S. was affiliated with the Cleveland Clinic during the time of this study.
Ethical Statement: The study was approved by institutional ethics board of Cleveland Clinic (No. IORG0000301).
References
- Ferlay J SI, Ervik M, Dikshit R, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013 [March 15, 2015]. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=colorectal
- National Comprehensive Cancer Network. Clinical Practice Guidelines (NCCN Guidelines): Colon Cancer (Version 2.2015) 2015 [March 15, 2015]. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1-9. [Crossref] [PubMed]
- Adam R, Haller DG, Poston G, et al. Toward optimized front-line therapeutic strategies in patients with metastatic colorectal cancer--an expert review from the International Congress on Anti-Cancer Treatment (ICACT) 2009. Ann Oncol 2010;21:1579-84. [Crossref] [PubMed]
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. [Crossref] [PubMed]
- Kennedy A. Radioembolization of hepatic tumors. J Gastrointest Oncol 2014;5:178-89. [PubMed]
- Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012;56:464-73. [Crossref] [PubMed]
- Cremonesi M, Chiesa C, Strigari L, et al. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front Oncol 2014;4:210. [Crossref] [PubMed]
- Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 2009;19:951-9. [Crossref] [PubMed]
- Raval M, Bande D, Pillai AK, et al. Yttrium-90 radioembolization of hepatic metastases from colorectal cancer. Front Oncol 2014;4:120. [Crossref] [PubMed]
- Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135-150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J Vasc Interv Radiol 2005;16:1641-51. [Crossref] [PubMed]
- Giammarile F, Bodei L, Chiesa C, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging 2011;38:1393-406. [Crossref] [PubMed]
- SIR-Spheres Training Program North Sydney, Australia: SIRTeX Medical Limited; [May 1, 2015]. Available online: http://foxfireglobal.sirtex.com/sites/foxfireglobal.sirtex.com/files/user/trn-rw-04_for_eu_au_nz_and_asia.pdf
- SIR-Spheres Microspheres Activity Calculator North Sydney, Australia: SIRTeX Medical Ltd.; [May 1, 2015]. Available online: http://apps01.sirtex.com/smac/
- TheraSphere Yttrium-90 Microspheres Instructions for Use. Surrey, United Kingdom: Biocompatibles UK Ltd; [May 1, 2015]. Available online: http://www.therasphere.com/physicians-package-insert/TheraSphere_IFU_EU_English_Rev7.pdf
- National Cancer Institute. Common Terminology Criteria for Adverse Events v4.03 2010 [May 27, 2015]. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- American College of Radiology (ACR). ACR-SIR Practice Parameter for Radioembolization with Microsphere Brachytherapy Device (RMBD) for Treatment of Liver Malignancies. 2014.
- Abbott AM, Kim R, Hoffe SE, et al. Outcomes of Therasphere Radioembolization for Colorectal Metastases. Clin Colorectal Cancer 2015;14:146-53. [Crossref] [PubMed]
- Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol 2015;6:134-42. [PubMed]
- Lewandowski RJ, Memon K, Mulcahy MF, et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival by era and chemotherapy. Eur J Nucl Med Mol Imaging 2014;41:1861-9. [Crossref] [PubMed]
- Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer 2009;115:1849-58. [Crossref] [PubMed]
- Seidensticker R, Denecke T, Kraus P, et al. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol 2012;35:1066-73. [Crossref] [PubMed]
- Bester L, Meteling B, Pocock N, et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol 2012;23:96-105. [Crossref] [PubMed]
- Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 2010;103:324-31. [Crossref] [PubMed]
- Cianni R, Urigo C, Notarianni E, et al. Selective internal radiation therapy with SIR-spheres for the treatment of unresectable colorectal hepatic metastases. Cardiovasc Intervent Radiol 2009;32:1179-86. [Crossref] [PubMed]
- Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol 2008;19:1187-95. [Crossref] [PubMed]
- Nace GW, Steel JL, Amesur N, et al. Yttrium-90 radioembolization for colorectal cancer liver metastases: a single institution experience. Int J Surg Oncol 2011;2011:571261.
- Saxena A, Kapoor J, Meteling B, et al. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol 2014;21:1296-303. [Crossref] [PubMed]
- Srinivas SM, Natarajan N, Kuroiwa J, et al. Determination of Radiation Absorbed Dose to Primary Liver Tumors and Normal Liver Tissue Using Post-Radioembolization (90)Y PET. Front Oncol 2014;4:255. [Crossref] [PubMed]
- Lea WB, Tapp KN, Tann M, et al. Microsphere localization and dose quantification using positron emission tomography/CT following hepatic intraarterial radioembolization with yttrium-90 in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol 2014;25:1595-603. [Crossref] [PubMed]
- Goin JE, Salem R, Carr BI, et al. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J Vasc Interv Radiol 2005;16:205-13. [Crossref] [PubMed]
- Kennedy AS, McNeillie P, Dezarn WA, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys 2009;74:1494-500. [Crossref] [PubMed]


