BRCA-associated protein 1 mutant cholangiocarcinoma: an aggressive disease subtype
Introduction
Cholangiocarcinoma (CCA) is an uncommon but aggressive malignancy of the biliary tract. Although CCA is reported to be more prevalent in Asia (secondary to endemic liver fluke infestation), the incidence of CCA is increasing worldwide (1). Around 4,000–5,000 cases of CCA are diagnosed each year in the United States, with an annual age-adjusted incidence rate of 0.85 per 100,000 (2-4). No effective screening tests for CCA are available, and a significant proportion of CCA patients present with locally advanced and/or metastatic disease, which precludes curative surgery and leads to a poor 5-year rate of survival after diagnosis (5). New strategies to enable earlier diagnosis, treatment, and prognosis are needed.
Information on genomic mutations and molecular pathways associated with CCA appears to have potential use in such strategies but is still evolving. Next-generation sequencing (NGS) studies have identified TP53, KRAS, ARID1A, IDH1, MCL1, PBRM1, ERBB2, SMAD4, FBXW7, and CDKN2A genes as being frequently aberrant in CCA (5). KRAS, TP53, and MAPK/mTOR aberrations in intrahepatic CCA are associated with worse prognosis (5). Genomic information may become increasingly important in the management of CCA and may explain why tumors with seemingly identical histopathology have significantly different clinical courses and responses to treatment.
Recent findings indicate that germline mutations in BAP1 (the gene encoding BRCA-associated protein 1) are associated with a higher incidence of melanoma, mesothelioma, breast cancer, ovarian cancer, lung cancer, and renal carcinoma (5). Furthermore, in comparison with malignancies not associated with BAP1 mutations, malignancies associated with BAP1 mutations develop in individuals at an earlier age (6,7). However, the clinical phenotype of BAP1-mutant CCA has been inadequately described in the literature. In this study, we explore the clinicopathological features and treatment outcomes of CCA harboring BAP1 mutation, which may help guide treatment decisions.
Methods
Patients selection
We retrospectively reviewed the records of CCA patients with BAP1 mutation treated at The University of Texas MD Anderson Cancer Center from January 1, 2009, to February 1, 2015. Patients with a histological diagnosis of CCA plus BAP1 mutation confirmed by NGS were identified. The electronic records of patients were used to collect and retrospectively analyze demographic, clinical, radiological, histopathological, and genetic characteristics; treatment received; time to progression; and survival. The study was approved by the Institutional Review Board.
Next-generation sequencing (NGS)
Genomic analysis for CCA was performed with NGS by Foundation Medicine (Cambridge, MA, UK) using archival fresh frozen paraffin-embedded tumor blocks of surgically resected or biopsied tumors. The library was constructed using 50–200 ng of DNA sheared to B100–400 bp before end repair, addition of deoxyadenylic acid, and ligation of indexed Illumina sequencing adaptors. Enrichment of target sequences was achieved by solution-based hybrid capture with custom Agilent SureSelect biotinylated RNA bait set. The genes selected for analysis were obtained from The Cancer Genome Atlas (TCGA) or the Catalogue of Somatic Mutations in Cancer. The selected libraries were sequenced on an Illumina HiSeq 2000 platform using 49,149 paired-end reads. Sequence data from genomic DNA were mapped onto the reference human genome (hg19) using the Burrows-Wheeler Aligner and processed using the open-source packages SAMtools, Picard, and Genome Analysis Toolkit (8,9). Point mutations were identified by a Bayesian algorithm, short insertions and deletions by local assembly, gene copy number alterations by comparing with process-matched normal controls, and gene fusions/rearrangements by clustering chimeric reads mapped to targeted introns (10).
Results
Patient demographic and clinical characteristics, histopathology findings, and stages of disease [according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, Seventh Edition 2010] (11) are listed in Table 1. There were 10 men and 12 women. Twenty patients had intrahepatic CCA and two had extrahepatic CCA. Sixteen patients had stage IV disease (Any T, Any N, and M1, where M1 is any distant metastasis), two had stage III disease, and four had stage II disease. Underlying hepatic diseases included cirrhosis (n=1), hepatitis C (n=1), and nonalcoholic steatohepatitis (n=1). Patients had various presenting symptoms, including chest and upper abdominal pain (n=8), elevated/abnormal liver enzymes (n=3), and weight loss (n=2). Twelve patients (56%) had poorly differentiated tumors and nine (41%) had moderately differentiated tumors. In four patients, the tumor was discovered as an incidental finding on a computed tomography (CT) scan. In one patient, the tumor was identified by CT performed for the surveillance of hepatitis C-associated liver disease. Diagnosis of CCA was made in all 22 patients by biopsy and histopathology and the presence of BAP1 mutation was identified by NGS.
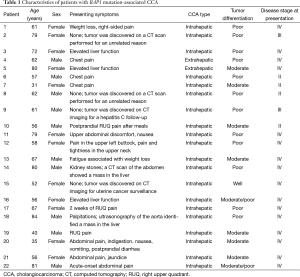
Full table
All patients underwent clinical disease staging by CT of the chest, abdomen, and pelvis. Tumor sizes, the presence or absence of vascular invasion and biliary dilatation, and distant metastases are listed in Table 2. Tumor size (greatest dimension) varied from 2 to 16 cm (mean, 8.5 cm). Seven patients had both vascular invasion and biliary dilatation. Twenty patients had liver metastases and 16 had nodal metastases. Thirteen patients had bone metastases.
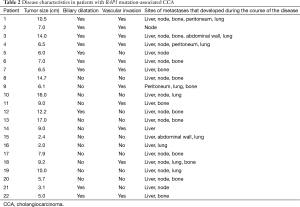
Full table
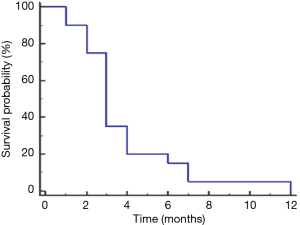
Treatment and time to progression and survival data are listed in Table 3. As of October 2015, six patients had died, and one was lost to follow-up. Therapeutic management varied according to the extent of disease and presence of comorbidities. Four patients presented with early-stage disease (stage II). Two patients, one with stage III and one with stage IV disease, underwent primary surgical resection followed by adjuvant chemotherapy and chemoradiotherapy, respectively, and two others, both with stage II disease, underwent neoadjuvant chemotherapy followed by surgery and postoperative chemotherapy. In all four patients, the disease radiologically progressed as per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (12) within 4 months of resection (Table 3). Eighteen patients had metastatic disease at presentation. Twelve of these patients underwent chemotherapy and the remaining six patients underwent chemoradiation (Table 3). All patients with metastasis had progressive disease; the mean time to progression was 3.8 months (Figure 1). First-line chemotherapy regimens administered included gemcitabine plus cisplatin (n=16); gemcitabine, cisplatin, and erlotinib (n=2); and gemcitabine plus capecitabine (n=1). Bone metastases was common (n=13; 59%) (Table 2).

Full table
The aggressive nature of CCA was particularly evident in two patients. Patient 6, who presented with poorly differentiated primary intrahepatic CCA, underwent neoadjuvant chemotherapy followed by extended left hepatectomy, but hepatic metastasis developed within 8 weeks after surgery. Patient 7, who presented with moderately differentiated primary intrahepatic CCA, underwent orthotopic liver transplantation, but the disease recurred within 8 months after the procedure.
Discussion
The objective of this study was to describe the clinical features of BAP1-mutant CCA to help guide treatment decisions. This case series had the largest number of CCA patients with BAP1 mutation described to date. Our findings show that BAP1-mutant CCA is an aggressive disease, with time to progression in our study being much lower than that reported for patients with advanced CCA treated with gemcitabine plus cisplatin (8 months in the Advanced Biliary Cancer trial) (13). As in uveal melanoma, wherein BAP1 mutation is seen more frequently (14), the presence of this mutation in the patients in our case series indicated poor prognosis at both surgically resectable and advanced stages of disease.
BAP1 encodes deubiquitylating enzymes and is found in association with multiprotein complexes that regulate key cellular pathways in the cell cycle, cellular differentiation, cell death, gluconeogenesis, and the DNA repair process (6,7). The chromosome region, wherein BAP1 is located, 3p21.1, is deleted in several malignancies (6,7). BAP1 acts as a tumor suppressor through chromatin remodeling, DNA repair, and ubiquitin-proteasome pathways. Germline mutation of BAP1 is associated with increased susceptibility to uveal melanoma, epithelioid atypical Spitz tumors, cutaneous melanoma, and mesothelioma (14,15).
The patients in our case series had no notable family history of CCA; therefore, the BAP1 mutations were likely somatic. Somatic BAP1 mutations, although rare, have been described in prostate, ovarian, colon, breast, and lung cancers and mesothelioma (7). Our finding that BAP1-mutant CCA follows an aggressive course is consistent with the findings of a study reporting that BAP1 mutations are associated with an aggressive metastatic phenotype in uveal melanoma and renal cell carcinoma and that depletion of BAP1 induces loss of differentiation ability and early dissemination in uveal melanoma (14,15).
Because BAP1 mutation is a chromatin-remodeling mutation, histone deacetylase inhibitors such as vorinostat and panobinostat may have activity against BAP1 mutation-associated disease (16-19). Some BAP1-associated tumors such as atypical Spitz tumors have abundant lymphocytic infiltrates, increasing the possibility that these tumors may respond to immunotherapy. Currently clinical trials are lacking for this orphan disease population. However, we must emphasize that the detection of BAP1 mutation does not necessarily indicate relevance of the mutation as a prognostic biomarker (or a potential therapeutic target). An integrated approach is required to expedite current efforts to identify prognostic markers and therapeutic targets for BAP1-mutant CCA.
Conclusions
Genomic sequencing can potentially identify distinct molecular subsets of CCA likely with prognostic and therapeutic implications. BAP1 mutation confers poor response to standard therapies; some genotype–phenotype correlations should be established in CCA as carriers of BAP1 mutations. Further extensive clinical, epidemiological, and functional studies are required to define the role of BAP1 and its interaction in CCA and to identify therapeutic targets in CCA with BAP1 mutation. Agents directed at these targets may be useful for the treatment of patients with BAP1-mutated CCA.
Acknowledgements
This work was supported by a grant from the Cholangiocarcinoma Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board (No. PA 13-0206)
References
- Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis 2008;12:131-50. ix. [Crossref] [PubMed]
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [Crossref] [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [Crossref] [PubMed]
- Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol 2007;102:1016-21. [Crossref] [PubMed]
- Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 2014;9:e115383. [Crossref] [PubMed]
- Carbone M, Yang H, Pass HI, et al. BAP1 and cancer. Nat Rev Cancer 2013;13:153-9. [Crossref] [PubMed]
- Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology 2013;45:116-26. [Crossref] [PubMed]
- Gnirke A, Melnikov A, Maguire J, et al. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol 2009;27:182-9. [Crossref] [PubMed]
- McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010;20:1297-303. [Crossref] [PubMed]
- Li H, Handsaker B, Wysoker A, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009;25:2078-9. [Crossref] [PubMed]
- American Joint Committee on Cancer Staging Manual, Seventh Edition. New York: Springer, 2010. Available online: https://cancerstaging.org
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410-3. [Crossref] [PubMed]
- Wiesner T, Obenauf AC, Murali R, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 2011;43:1018-21. [Crossref] [PubMed]
- Landreville S, Agapova OA, Matatall KA, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res 2012;18:408-16. [Crossref] [PubMed]
- Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013;45:1470-3. [Crossref] [PubMed]
- Ma X, Ezzeldin HH, Diasio RB. Histone deacetylase inhibitors: current status and overview of recent clinical trials. Drugs 2009;69:1911-34. [Crossref] [PubMed]
- Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641-5. [Crossref] [PubMed]


