Dihydro pyrimidine dehydrogenase deficiency in patients treated with capecitabine based regimens: a tertiary care centre experience
Introduction
5FU and capecitabine based chemotherapeutic regimens form the backbone of chemotherapy in multiple GI malignancies, both in the adjuvant and metastatic settings (1). 5FU and capecitabine form the most important component of treatment in colorectal malignancies (2) as well as GEJ and stomach tumours (3). Numerous serious adverse side effects have been reported with fluoropyrimidine treatment, including myelosuppression, cardiac toxicity, mucositis, hand-foot syndrome (HFS), and diarrhoea. Capecitabine has been increasingly used because of the convenience of its oral administration and mimicry of the infusional 5FU regimen along with potential therapeutic advantages over bolus 5FU. In addition, capecitabine is better tolerated by patients, who reported fewer cases of stomatitis, alopecia, neutropenia, diarrhoea, and nausea, but higher incidence of HFS, with capecitabine as compared to 5FU (4).
The variation in responses and adverse effects profile with capecitabine is related to the complex interplay between anabolism and catabolism of this molecule and genetic differences between individuals (5,6). While the anabolic pathways probably directly mediate the cytotoxic effects, the catabolic route likely plays a more important role in toxicity as more than 80% of capecitabine is catabolized by the rate limiting enzyme dihydropyrimidine dehydrogenase (DPD) enzyme (6). The DPD deficiency syndrome, which is a familial syndrome as a result of the allelic mutations within the DPYD gene, classically consists of early onset and exaggerated toxicities including mucositis, diarrhoea, myelosuppression, HFS, neutropenia, and rarely, but characteristically, neurologic deficits (7). While DPD deficiency has consistently been linked with toxicity on 5FU administration (8,9), controversy exists on whether all patients planned for 5FU/capecitabine should be tested for DPD activity or mutation prior to treatment and what is the optimum method for detection of DPD activity (10,11). Data on capecitabine is especially scarce, with only a few case reports on the pattern of toxicity with capecitabine (9). There is also evidence of subtle differences between 5FU and capecitabine in terms of drug interactions and side-effect profile, which is hampered by the absence of definite data regarding capecitabine and DPD deficiency (12,13).
While DPD mutation testing was not used routinely in patients treated at our centre, based on certain criteria of clinical toxicity, we tested for DPD mutation in patients on capecitabine who developed these pre-defined toxicities. Our major aim with this analysis was to enunciate clinical testing criteria, as well as record the incidence and DPD mutation status of prospectively collected data for patients who were evaluated for DPD mutation status while on capecitabine based regimens. To the best of our knowledge, this is the largest data set regarding DPD mutation status in Indian patients with GI cancers treated with capecitabine.
Methods
All patients treated in the Medical Oncology GI unit with capecitabine based regimens between June 2013 to June 2015 were included in this analysis. Regimens included the following at standard recommended doses:
- EOX: epirubicin/oxaliplatin/capecitabine;
- DOX: docetaxel/oxaliplatin/capecitabine;
- Capecitabine with concurrent Radiotherapy;
- CAPOX: capecitabine + oxaliplatin;
- Capecitabine single agent;
- CAPIRI: capecitabine/irinotecan.
Patients who developed at least 2 of the following concurrent toxicities in cycle 1 were selected for DPD mutation analysis:
- Grade 3 or above mucositis;
- Grade 3 or above diarrhoea;
- Grade 3 or above myelosuppression;
- Grade 3 HFS.
These criteria were selected by (VO) based on data extrapolated from DPD mutations in relation to toxicities seen with 5FU in colorectal cancer patients (14,15).
Patients who developed the above mentioned toxicities had dose reductions or modifications for the subsequent chemotherapy cycle, C2, based on individual treating clinician choice, while awaiting DPD testing reports. Once test reports were available, dose modifications were mostly based on clinical pharmacological consortium guidelines for future cycles of chemotherapy (16).
Clinical data collection
For the purposes of this study, A Sahu and A Ramaswamy collected the demographic data, toxicity, DPD mutation testing details, dose reduction and details of change in regimen from a prospectively maintained GI database and electronic medical record system. Toxicity was assessed by treating physician and independently reconfirmed by (VO) prior to documentation in patients where (VO) was not the primary attending physician.
DPD testing
The DPD mutation testing was done on peripheral blood in a commercial laboratory by PCR-sequencing method. Eleven mutations are detectable by this assay and include splice site point mutation IVS14+G/A, 85 T/C, 61 T/C, 496 A/G, 601 A/C, 632 A/G, 1601 G/A, 1627 A/G, 1678 T/G, 2194 G/A and 2846 A/T, respectively.
DPD levels/activity could not be measured. There was no interaction between lab personnel conducting the DPD testing and patients tested, i.e. lab personnel were blinded to patient data and outcomes.
Statistical analysis
SPSS version 20 was used for analysis. Descriptive statistics including median, frequency and percentage for categorical variables is used to describe age, sex distribution, cancer primary distribution, chemotherapy regimen type, toxicity profile and DPD mutation type. Differences in toxicity profile between cycle 1 and cycle 2 after dose modification was compared with the χ2 test. The predictive value of clinical toxicity based criteria was tested by the following formula.
Predictive value = (number of patients with DPD mutation leading to toxicity ×100)/(number of patients in whom DPD mutation was suspected on the basis of clinical toxicity criteria).
Results
Baseline characteristics
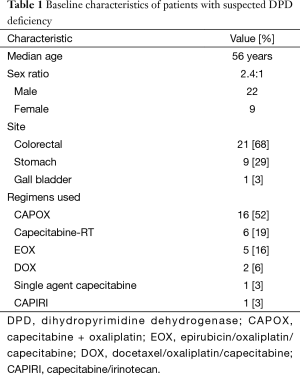
A total of 506 patients were treated in the Gastrointestinal Medical Oncology unit of our institution with capecitabine containing regimens during the period from June, 2013 to June, 2015. Thirty one patients developed at least two grade 3 and grade 4 toxicities during cycle 1 of capecitabine in this period, as per predefined clinical criteria. The median age of this cohort was 56 years (range, 26–67 years), with 22 males (71%) and 9 females (29%) in this cohort (Table 1). Twenty-one patients with colorectal cancers, 9 patients with stomach cancers and one patient with carcinoma gall bladder comprised this cohort. It was most commonly seen with the CAPOX regimen (52%) (Table 1). The distribution of toxicities in the whole cohort is captured in (Table 2).
Full table

Full table
Predictive value of clinical toxicity for DPD testing and mutation status
Of the 31 patients with suspected DPD deficiency, 3 patients declined testing. Of the 28 patients who underwent testing, 22 were detected to have a DPD mutation causing deficient or absent DPD enzyme activity (Table 2). Overall, 31 of 506 (6%) patients treated with capecitabine had two or more grade 3/4 life threatening complications and 22 of 506 (4.3%) were DPD mutant. The predictive value of clinical toxicity in predicting DPD mutation test positivity was 78.5%.
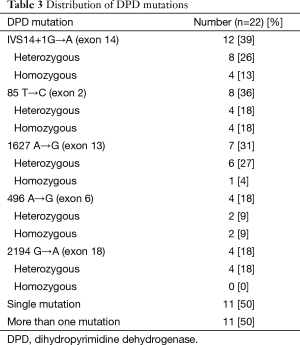
The commonest mutation seen was the splice-site mutation IVS14+1G→A, seen in 12 patients (39%), with 4 patients (13%) having heterozygosity. Other mutations seen were 85 T→C in 8 (36%), 1627 A→G in 7(32%), 496 A→G in 4 (18%) and 2194 G→A in 4 (18%) patients, respectively. Eleven of 22 patients (50%) had more than 1 mutation on testing (Table 3).
Full table
Toxicity in DPD mutated patients after dose modification
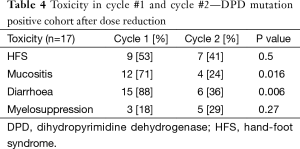
In the cohort of 22 patients who tested positive for DPD mutation, 4 patients were permanently discontinued chemotherapy, one patient had chemotherapy shifted to irinotecan based regimen, while 17 patients had dose modification of capecitabine to 50% of original dosage for cycle 2 of chemotherapy (Table 4).
Full table
In the 17 patients who underwent dose modification of capecitabine in cycle 2, HFS, mucositis and diarrhoea were reduced, while myelosuppression was increased, though not statistically significant. The incidence of mucositis and diarrhoea fell from 71% to 24% (P=0.016) and 88% to 36% (P=0.006) respectively, which was statistically significant. While the incidence of HFS was also reduced, this was not statistically significant (P=0.5). Five (29%) DPD mutant patients did not have any toxicity after dose reduction.
Discussion
DPD enzyme is the rate limiting step of 5 FU/capecitabine catabolism and deficiency in this enzyme is a well-recognized cause of approximately half the toxicity associated with 5FU infusions (15,17). A number of mutations and polymorphisms have been identified in the genomic structure of the DPD gene, with a few resulting in decreased activity of DPD (18-20). The most common clinically relevant mutation, worldwide is the IVS14+1G→A, in which a G to A mutation in the invariant GT splice donor site leads to the skipping of exon 14 immediately upstream of the mutated splice donor site in the process of DPD pre-mRNA splicing (17). Besides mutations, multiple other factors may contribute to the differences in incidence of toxicity with 5FU, including sex, race, nutritional parameters, etc. (21-24). Based on our analysis, at least 4.3% (22 out of 506) of Indian patients with GI cancers are positive for DPD mutation, with a homozygosity rate of 1.75% (9 out of 506). The commonest mutation seen in this study was the IVS14+1G→A mutation, consistent with worldwide data. While overall rates of DPD mutation positivity are comparable to previous published data recording 3–5%, the rates of homozygosity are markedly higher (7). Whether this is a difference that can be attributed to ethnic differences between the Indian populations and previously studied Caucasian cohorts or a higher prevalence in Indian GI cancer patients requires further evaluation in a much larger sample size in view of the clinical implications of the same. We were unable to find any population or cancer based studies regarding DPD status in Indian patients (PubMed search).
Capecitabine is an oral fluoropyrimidine that is an example of rational drug design to allow for selective 5FU activation in tumor tissue (25). It requires enzymatic conversion via a 3 step pathway, the final step of which occurs in tumor tissue by conversion of 5'-deoxy-5-fluorouridine to 5FU by the enzyme thymidine phosphorylase. This enzyme is present in much larger concentrations intratumor than in plasma or normal tissue (26,27), potentially improving efficacy and safety. Analysis of large scale clinical data, indeed, have shown similar efficacy and lesser toxicities with capecitabine when compared to 5FU, with the exception of an increased incidence of HFS (4,12,28). Based on these advantages, capecitabine is considered an acceptable substitute for 5FU in view of equivalent or improved efficacy, decreased toxicity and ease of administration. However, there is little experience with capecitabine in the setting of DPD deficiency, with a majority of the information coming from case reports (9). Our analysis suggests that despite a potential theoretical pharmacological benefit of capecitabine over 5FU, patients with DPD mutation, treated with capecitabine develop high rates of grade 3 and grade 4 toxicities. As expected, mucositis and diarrhoea predominated, but there were no cases with neurotoxicity. The clinical criteria we used as a trigger for DPD mutation testing seems reasonably accurate, with a predictive value of 78.5%. The addition of HFS as a clinical criteria, in additional to the traditional clinical indicators of mucositis, diarrhoea and neurotoxicity, for DPD mutation testing in patients on capecitabine may help us detect more patients with DPD mutation in view of its increased incidence with capecitabine as compared to 5FU. The importance of clinical criteria is magnified in our setting where we do not possess DPD mutation testing in house as yet. With the jury still out on whether all patients to be started on 5FU/capecitabine treatment should undergo a priori testing for DPD mutation or enzyme levels, clinical guidelines for testing need further validation.
Dose adjustments in cycle 2 were done initially, according to treating physician’s choice in our analysis. Reasons for this were 2 fold-primarily, because the results of the DPD testing took an average of 2 weeks to return. This resulted in potential delays in treatment, due to which individual physicians took a decision to stop capecitabine (as in 4 patients), change to irinotecan (1 patient) or modify doses by 50% (17 patients). Secondly, while the clinical pharmacology consortium guidelines provide general guidelines for stopping treatment or dose reduction, they are not completely accurate and more arbitrary than evidence based. As seen in this study, despite dose reduction, significant grade 3 and grade 4 toxicities, still occurred in patients (16). This may also be possible due to patients with a homozygous mutation, being rechallenged with capecitabine. This is a major downside of not waiting for the DPD mutation test results as there is evidence to suggest complete cessation of 5FU/capecitabine in certain homozygous mutations, i.e., IVS14+1G→A (17,18). However, what is evident is that dose reductions do lead to a significant decrement in specific toxicities, as seen by the statistically significant reduction in mucositis and diarrhoea, thereby allowing continuation of chemotherapy. Decisions regarding dose reduction and stoppage however, has to be tempered keeping in mind results of a recent analysis (29), which has suggested a correlation between toxicity and efficacy in colorectal cancer patients being treated with adjuvant 5FU based chemotherapy. Any grade of neutropenia, mucositis and nausea/vomiting predicted for DFS benefit, while any grade of nausea/vomiting predicted for OS benefit as well. Those patients experiencing no predefined toxicity had the worst outcomes. This coupled with the lack of alternate chemotherapeutic regimens in the adjuvant setting for colorectal cancer setting makes choosing therapy a perplexing decision.
Our study is the first of its kind in India and throws up some interesting points. It suggests that DPD mutation homozygosity may have a higher incidence in Indian patients with GI malignancies and this requires further studies. Clinical guidelines may help predict with reasonable accuracy the requirement for reflex testing for DPD mutation status. Capecitabine has a similar adverse effect profile as 5FU in the setting of DPD mutation and this needs to be kept in mind while managing toxicities with this drug. In view of the high incidence of life—threatening toxicities with capecitabine and potentially higher incidence of homozygosity in Indian patients, it may be prudent to consider upfront DPD mutation testing by a validated method prior to beginning 5FU/capecitabine based therapy.
The drawbacks of this study include a lack of comparison between outcomes in the DPD mutated cohort versus non mutated cohort. We were unable to get upfront DPD mutation testing in our patients due to lack of in house testing. This coupled with the lag time in procuring tests reports may result in patients with homozygous mutations being rechallenged with 5FU/capecitabine, which is not advisable, despite no formal recommendations regarding the same. The PCR method used by the referred lab tested for 11 mutations and may have missed other clinically significant mutations.
Conclusions
There is an unmet need for estimating prevalence of DPD mutations in the Indian population, especially in GI cancer patients. DPD deficiency should be kept in mind when treating complications with capecitabine based chemotherapeutic regimens. Clinical toxicity criteria need further evaluation and validation in a setting where formal recommendations regarding upfront testing for DPD status do not exist.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Grem JL. 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs 2000;18:299-313. [Crossref] [PubMed]
- Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. [Crossref] [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Van Cutsem E, Twelves C, Cassidy J, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 2001;19:4097-106. [PubMed]
- Heggie GD, Sommadossi JP, Cross DS, et al. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res 1987;47:2203-6. [PubMed]
- Diasio RB, Beavers TL, Carpenter JT. Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J Clin Invest 1988;81:47-51. [Crossref] [PubMed]
- Etienne MC, Lagrange JL, Dassonville O, et al. Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol 1994;12:2248-53. [PubMed]
- Importance of dihydropyrimidine dehydrogenase (DPD) deficiency in patients exhibiting toxicity following treatment with 5-fluorouracil. (Internet). Cited 2015 Oct 10. Available online: https://www.docphin.com/research/article-detail/15393408/PubMedID-11384742/Importance-of-dihydropyrimidine-dehydrogenase-DPD-deficiency-in-patients-exhibiting-toxicity-following-treatment-with-5-fluorouracil
- Saif MW, Diasio R. Is capecitabine safe in patients with gastrointestinal cancer and dihydropyrimidine dehydrogenase deficiency? Clin Colorectal Cancer 2006;5:359-62. [Crossref] [PubMed]
- van Kuilenburg AB, van Lenthe H, Zoetekouw L, et al. HPLC-electrospray tandem mass spectrometry for rapid determination of dihydropyrimidine dehydrogenase activity. Clin Chem 2007;53:528-30. [Crossref] [PubMed]
- Ahluwalia R, Freimuth R, McLeod HL, et al. Use of pyrosequencing to detect clinically relevant polymorphisms in dihydropyrimidine dehydrogenase. Clin Chem 2003;49:1661-4. [Crossref] [PubMed]
- Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 2001;19:2282-92. [PubMed]
- Twelves C, Boyer M, Findlay M, et al. Capecitabine (Xeloda) improves medical resource use compared with 5-fluorouracil plus leucovorin in a phase III trial conducted in patients with advanced colorectal carcinoma. Eur J Cancer 2001;37:597-604. [Crossref] [PubMed]
- High prevalence of the IVS14 + 1G>A mutation in the dihydropyrimidine dehydrogenase gene of patients with severe 5-fluorouracil-associated toxicity. Cited 2015 Oct 10. Available online: http://journals.lww.com/jpharmacogenetics/Fulltext/2002/10000/High_prevalence_of_the_IVS14___1G_A_mutation_in.7.aspx
- van Kuilenburg AB, De Abreu RA, van Gennip AH. Pharmacogenetic and clinical aspects of dihydropyrimidine dehydrogenase deficiency. Ann Clin Biochem 2003;40:41-5. [Crossref] [PubMed]
- Caudle KE, Thorn CF, Klein TE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther 2013;94:640-5. [Crossref] [PubMed]
- van Kuilenburg AB, Haasjes J, Richel DJ, et al. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res 2000;6:4705-12. [PubMed]
- Johnson MR, Yan J, Shao L, et al. Semi-automated radioassay for determination of dihydropyrimidine dehydrogenase (DPD) activity. Screening cancer patients for DPD deficiency, a condition associated with 5-fluorouracil toxicity. J Chromatogr B Biomed Sci Appl 1997;696:183-91. [Crossref] [PubMed]
- Van Kuilenburg AB, Vreken P, Abeling NG, et al. Genotype and phenotype in patients with dihydropyrimidine dehydrogenase deficiency. Hum Genet 1999;104:1-9. [Crossref] [PubMed]
- Collie-Duguid ES, Etienne MC, Milano G, et al. Known variant DPYD alleles do not explain DPD deficiency in cancer patients. Pharmacogenetics 2000;10:217-23. [Crossref] [PubMed]
- Chansky K, Benedetti J, Macdonald JS. Differences in toxicity between men and women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer 2005;103:1165-71. [Crossref] [PubMed]
- McCollum AD, Catalano PJ, Haller DG, et al. Outcomes and toxicity in african-american and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst 2002;94:1160-7. [Crossref] [PubMed]
- Saif MW, Mattison L, Carollo T, et al. Dihydropyrimidine dehydrogenase deficiency in an Indian population. Cancer Chemother Pharmacol 2006;58:396-401. [Crossref] [PubMed]
- Stenram U. 5-FU toxicity and nutritional deficiencies. Br J Cancer 1993;67:1157. [Crossref] [PubMed]
- Saif MW, Eloubeidi MA, Russo S, et al. Phase I study of capecitabine with concomitant radiotherapy for patients with locally advanced pancreatic cancer: expression analysis of genes related to outcome. J Clin Oncol 2005;23:8679-87. [Crossref] [PubMed]
- Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998;34:1274-81. [Crossref] [PubMed]
- Saif MW, Katirtzoglou NA, Syrigos KN. Capecitabine: an overview of the side effects and their management. Anticancer Drugs 2008;19:447-64. [PubMed]
- Cassidy J, Twelves C, Van Cutsem E, et al. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol 2002;13:566-75. [Crossref] [PubMed]
- Soveri LM, Hermunen K, de Gramont A, et al. Association of adverse events and survival in colorectal cancer patients treated with adjuvant 5-fluorouracil and leucovorin: Is efficacy an impact of toxicity? Eur J Cancer 2014;50:2966-74. [Crossref] [PubMed]


