Targeting stromal microenvironment in pancreatic ductal adenocarcinoma: controversies and promises
Introduction
Pancreatic cancer is a devastating disease, which is the fourth leading cause of cancer related death in the Unites States (1). The use of intensive cytotoxic regimen such as FOLFIRINOX has almost doubled the survival but the 2-year survival rate for patients with metastatic pancreatic ductal adenocarcinoma (PDA) is dismally <20% (2). Microscopically, PDA is characterized by thick desmoplastic stromal matrix surrounding islands of cancer cells. It is increasingly realized that the pancreatic stroma plays an active role in carcinogenesis, progression, metastasis, mediating drug resistance, and immunosuppression (3). PDA stroma is highly heterogeneous consisting of stellate cells [pancreatic stellate cells (PSCs)] or carcinoma-associated fibroblasts (CAFs) in activated form, microvasculature, nerves, inflammatory infiltrate and acellular extracellular matrix (ECM). Encouraging results from preclinical studies targeting the PDA stroma, such as using hyaluronidase to improve cytotoxic delivery and CD40 agonist to modulate immune response, renewed the field’s enthusiasm and led to a number of clinical trials evaluating stromal-targeting therapy in PDA. However, the failure of hedgehog (Hh) inhibitor to improve patient outcome during clinical evaluation despite positive preclinical results is humbling and demonstrated the paucity in our understanding of the complex PDA biology (4-7). Here, we review the stromal-targeting strategies currently in (or, near-to) clinical evaluation and their preclinical rationales (Table 1).
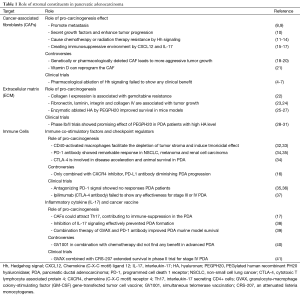
Full table
The biology of stromal cells in PDA
The prominent feature of PDA is dense desmoplastic stroma, sometimes comprising up to 80% of tumor mass (42). As early as a decade ago, researchers found that highly heterogeneous components of the stroma, consisting of immune cells, CAF, ECM as well as varieties of proteins, enzymes, growth factors and cytokines, form a sophisticated network interacted with tumor cells (43). Depletion of stromal component has been associated with improved prognosis in some animal models and early-stage clinical trials (11,32,42). α-smooth muscle actin (αSMA) secreted by CAF is confirmed as a negative prognostic factor in PDA (44). Whereas, the current outcome of clinical trials targeting pancreatic stroma does not meet the high expectation. More importantly, recently published several studies provide new explanations for the failure of trials, suggesting ablation of the stroma may lead to poorly differentiated tumors and accelerate PDA progression (18-21). These conflicting evidences prompt us to revisit the role of stromal cell.
Cancer-associated fibroblasts (CAFs)
CAFs have been found to promote tumor progression and metastasis in pancreatic cancer (8,9). The stromal cells mediate the formation of ECM that protects cancer stem cells, secrets growth factors promoting tumor proliferation, and interrupt immune-surveillance resulting in immunosuppressive tumor microenvironment (10,15) (Figure 1). In turn, CAFs’ biology is positively regulated by PDA cells (43).
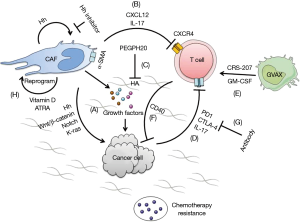
The stromal cells were found in preclinical studies to impede the penetration of anti-cancer drugs resulting in inadequate cell kills (11,12). Hh signaling was implicated as a key regulator of tumor-stromal interaction in PDA (13). Using genetic KPC mice model (KrasLSL.G12D/+; p53R172H/+; PdxCretg/+), Olive et al. (11) found that interrupting Hh signaling using IPI-926 (saridegib) ablate stromal CAFs leading to transient increase in intratumoral vascular density and increased intratumoral gemcitabine level, resulting in better cytotoxic effect. Similar results were observed using a different Hh inhibitor (AZD8542) (14). However, the clinical trials evaluating Hh inhibitors in PDA failed to demonstrate clinical benefit despite such positive preclinical results. The phase II randomized study using gemcitabine with/without IPI-926 was stopped early due to increased mortality or not showing benefit (4,6,45). Similarly, a single-arm phase II trial (NCT01195415) of GDC-0449 (vismodegib) with gemcitabine was not superior in metastatic PDA compared to gemcitabine alone in historical control (7). The failure of clinical trials to replicate the preclinical success was puzzling and reasons suggested include limitations in the mouse models, chronic versus acute ablation of stromal cells by Hh inhibitors, and off-target effects of the drugs (46). In addition, there was an absence of potential predictive biomarkers such as stromal characteristics to guide clinical trial design (47).
Interestingly, several recent preclinical reports contradicted earlier studies suggesting that Hh-mediated stromal response restrained tumorigenesis and ablation of which was detrimental in PDA. Özdemir et al. deleted αSMA myofibroblasts by crossing PtflaCre/+; KrasLSL-G12D/+; Tgfbr2flox/flox (PKT) mice, demonstrated that the depletion of myofibroblast yielded undifferentiated and more invasive PDA (19). Similar results were also observed in KPC mice crossed with αSMA-transgenic mice (19). The decreased elastic content in PDA did not improve intratumoral gemcitabine concentration. In contrast, it was correlated with reduced survival and confirmed that actually desmoplasia protected the host. Separately, Rhim et al. specifically deleted Sonic hedgehog (Shh) ligand expression in mice PDA stroma by crossing Pdx1Cre/+; KrasLSL-G12D/+; p53fl/+; Rosa26LSL-YFP/+ (PKCY) with Shhfl/fl mice. Surprisingly, such Shh-deficent tumors were more aggressive, exhibiting increased vascularity, heightened proliferation and these were recapitulated using Hh inhibitors in KPC mice (20). Lee et al. showed that in three distinct genetically engineered mice models, Hh pathway inhibition suppressed stromal desmoplasia and accelerated growth of the epithelial elements; whereas, activation of Hh signaling caused stromal hyperplasia and reduced epithelial proliferation leading restraint on tumorigenesis (18).
Other novel stromal modulating therapies had been explored preclinically. Sherman et al. reported activation of vitamin D receptor (VDR) could re-program PSCs to a more quiescent and less tumor-supporting state that potentially countered PDA progression (21). In transgenic mice models, VDR activation reduced inflammatory markers and fibrosis, and increasing intratumoral gemcitabine level, Froeling et al. showed that treatment with all-trans retinoic acid (ATRA) induced CAFs quiescence, leading to reduced cancer cell proliferation and invasion, and increased apoptosis via Wnt-β-catenin signaling (48).
Acellular extracellular matrix (ECM)
The acellular part of PDA stroma is composed of proteins, polysaccharides and peptides. Secreted by CAFs, these stromal elements not only provide structural support but are also involved in differentiation, remodeling and carcinogenesis. Collagen I was shown to promote gemcitabine resistance in vitro (22,23). It also interacted with collagen IV and integrins on the surface of PDA cancer cells, and is vital for proliferation, maintenance of migratory phenotype, and avoiding apoptosis (24). Other potential ECM remodeling genes differentially expressed in PDA stroma included matrix metalloproteinase 3, collagen type IVα1 and syndecan-2 (49), though their role in PDA tumor-stromal interaction remains unclear for now.
Hyaluronan (HA) is a polysaccharides found in HA stromal matrix. High HA level in PDA increased interstitial fluid pressure (IFP) in tumor, creating substantial barriers to perfusion that attenuate the effects of anti-cancer drugs (25,50). In KPC and KC mice models, treatment using PEGylated human recombinant PH20 hyaluronidase (PEGPH20) ablated stromal HA that led to IFP normalization and re-expansion of collapsed tumor vasculature without increasing the microvessel density (26). When combined with gemcitabine, PEGPH20 significantly enhanced drug penetration throughout the tumor tissues, inhibited tumor growth and extended the mice survival. Similar result was reported by Jacobetz et al. (27). Elevated HA level was also found in metastatic PDA lesions, suggesting that HA targeting might also benefit in metastatic disease.
In stage I/IB clinical trials, PEGPH20 in combination with gemcitabine achieved partial metabolic responses by FDG-PET/CT in 4 out of 5 pancreatic cancer patients using PEGPH20 (28), and particularly showed promising activity in those with high HA levels (29,30). The randomized, phase II trials evaluating PEGPH20 in combination with nab-paclitaxel and gemcitabine (NCT01839487) and S1313 trial (NCT01959139) assessing PEGPH20 in combination with modified FOLFIRINOX for previously untreated metastatic PDA are ongoing presently. Preliminary result revealed that PEGPH20 + nab-paclitaxel + gemcitabine offered greater overall response rate (ORR) and progression free survival (PFS) in patients with high HA status (31).
Immune cells
Broad repertoire of immune cells has been involved in pancreatic cancer stroma. However, PDA creates a hypoxic and highly immunosuppressive environment which is resistant to inhibitory cytokines and immune cells anti-tumor effect (10,51). Recent studies focus on recruiting anti-tumor cells or cytokines to restore their responses.
Immune co-stimulatory factor and checkpoint regulators
CD40 is a cell surface molecule that is a member of the tumor necrosis factor (TNF) receptor family, and participates in immune regulation and mediates tumor apoptosis (52). CD40 was found to be a key regulator in the development of T cell-dependent anti-tumor immunity (53) though recent report showed that CD40-mediated anti-tumor were macrophage dependent (32). Using KPC mice model, Beatty et al. showed that both treatments using CD40 monoclonal antibody combined with gemcitabine caused tumor regression in 30% of mice, far superior to gemcitabine alone (32). They further showed that the tumor regression was mediated by macrophage that facilitated the depletion of tumor stroma. CP-870, 893, a monoclonal antibody, is a CD40 agonist that was evaluated in combination with gemcitabine in a phase I trial in patients with advanced PDA (33). Four of twenty-two patients (18%) achieved partial response. Interestingly, after-treatment tumor biopsy showed an absence of tumor-infiltrating lymphocyte and abundant macrophages.
Activation of programmed cell death 1 receptor (PD-1) by binding with PD-1 ligands (PD-L1 and PD-L2) suppresses the T-cell activity and makes cancer cell “invisible” to the immune system. Both PD-1 and PD-1 ligands are expressed in PDA and had been associated with poor prognosis (36). Although early clinical trial of anti-PD-1 antibody achieved response in non-small cell lung cancer, melanoma and renal cell carcinoma (34), but not in PDA (35,36). Treatment against cytotoxic T lymphocyte associated protein 4 (CTLA-4), another costimulatory signaling, improved survival in transgenic mice PDA model (19). A phase II trial using ipilimumab (anti-CTLA-4 antibody) failed to achieve tumor response by RECIST criteria in advanced PDA though delayed shrinkage was observed in one patient with continued dosing (37).
The reasons for the failure of PD-1 and CTLA-4 to achieve tumor response in PDA are unclear. The clue might come from another immune molecule. Chemokine (C-X-C motif) ligand 12 (CXCL12) is a ligand for chemokine (C-X-C motif) receptor 4 (CXCR4). CAFs expressing fibroblast activation protein (FAP) created immunosuppressive environment in PDA via secretion of CXCL12. Administering CXCR4 inhibitor, plerixafor (AMD3100), induced rapid T-cell response and acted synergistically with PD-L1 antibody to greatly diminish cancer cells in KPC mice (16). As such, therapies combining immune checkpoints inhibitors with an agent that reverse the immunosuppression in the tumor microenvironment like CXCR4 inhibitor have potential therapeutic application in PDA. This hypothesis is currently in clinical evaluation (NCT02301130).
Inflammatory factors
Inflammatory cytokines had been shown to promote tumorigenesis. Inflammatory cells such as regulatory T cells (Tregs) and tumor-associated macrophage (TAM) subtype M2 could attenuate intra-tumor immunity by secreting interleukin 10 (IL-10) and transforming growth factor β (TGFβ) (54). In addition, tumor cells and CAFs could attract interleukin-17 (IL-17) secreting CD4+ cells (Th17) via TGFβ secretion, contributing to immune-suppression in the PDA tumor microenvironment (17). McAllister et al. illustrated that IL-17 stimulated infiltration of IL-17-expressing T cells drive tumor progression and the disruption of IL-17 signaling prevented PDA formation in preclinical studies (38). Antibodies against IL-17 signaling pathway (brodalumab and ixekizumab) are currently evaluated by clinical trials for treating psoriasis. Therapies utilizing IL-17 inhibitors may hold promise in PDA.
Cancer vaccine
GVAX pancreas is a cancer vaccine generated from pancreatic cancer cell line and has been modified to express granulocyte-macrophage colony-stimulating factor (GM-CSF), which attract dendritic cell (DC) to present tumor antigen to T cells. The effect of GVAX can be amplified by co-administration with CRS-207, an attenuated listeria monocytogenes, as a boost vaccine to express mesothelin (marker of mesothelioma, ovarian and pancreatic cancer) (55). In phase II trial for stage IV PDA patients, GVAX/cyclophosphamide combined with CRS-207 extended 2 months survival compared to GVAX/cyclophosphamide (6 vs. 4 months) (41). In murine studies, GVAX treatment significantly upregulate PD-L1 expression and combination therapy of GVAX and PD-1 antibody improved survival (39). In contrast, simultaneous telomerase vaccination (GV1001) in combination with chemotherapy did not find any benefit in patient with advanced PDA in previously untreated patients (40). Accordingly, further evaluation of the efficacy of cancer vaccination is warranted in the future studies.
Conclusions
The increasing understanding of the tumor-stromal interactions in PDA has engendered many novel approaches to targeting the tumor stroma. The complexity and dynamic nature of PDA microenvironment became more apparent following the failure of earlier attempts such as that targeting the Hh signaling, suggesting that more robust preclinical/translational studies and novel clinical trial designs are needed. Currently, there are a number of promising stromal-targeting approaches under clinical investigation that may potentially groundbreaking. Recent report from Moffitt et al. utilized elegant bioinformatics methods to distinguish gene signatures from pancreatic tumor cells and stromal cells that independently predict patient outcome (56). Such advances will help tailor personalized treatments in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Erkan M, Hausmann S, Michalski CW, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol 2012;9:454-67. [Crossref] [PubMed]
- Richards DA, Stephenson J, Wolpin BM, et al. A phase Ib trial of IPI-926, a hedgehog pathway inhibitor, plus gemcitabine in patients with metastatic pancreatic cancer. J Clin Oncol 2012;30:abstr 213.
- Ko AH, LoConte NK, Kantoff E, et al. A phase Ib trial of FOLFIRINOX plus saridegib, an oral hedgehog (Hh) inhibitor, in pts with advanced pancreatic cancer (PDAC). J Clin Oncol 2012;30: abstr 3105.
- Stephenson J, Richards DA, Wolpin BM, et al. The safety of IPI-926, a novel hedgehog pathway inhibitor, in combination with gemcitabine in patients (pts) with metastatic pancreatic cancer. J Clin Oncol 2011;29: abstr 4114.
- Kim EJ, Sahai V, Abel EV, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res 2014;20:5937-45. [Crossref] [PubMed]
- Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res 2008;68:2085-93. [Crossref] [PubMed]
- Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 2013;144:1210-9. [Crossref] [PubMed]
- Rucki AA, Zheng L. Pancreatic cancer stroma: understanding biology leads to new therapeutic strategies. World J Gastroenterol 2014;20:2237-46. [Crossref] [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [Crossref] [PubMed]
- Waghray M, Yalamanchili M, di Magliano MP, et al. Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol 2013;29:537-43. [Crossref] [PubMed]
- Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 2003;425:851-6. [Crossref] [PubMed]
- Hwang RF, Moore TT, Hattersley MM, et al. Inhibition of the hedgehog pathway targets the tumor-associated stroma in pancreatic cancer. Mol Cancer Res 2012;10:1147-57. [Crossref] [PubMed]
- Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010;330:827-30. [Crossref] [PubMed]
- Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212-7. [Crossref] [PubMed]
- He S, Fei M, Wu Y, et al. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci 2011;12:7424-37. [Crossref] [PubMed]
- Lee JJ, Perera RM, Wang H, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci U S A 2014;111:E3091-100. [Crossref] [PubMed]
- Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719-34. [Crossref] [PubMed]
- Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25:735-47. [Crossref] [PubMed]
- Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014;159:80-93. [Crossref] [PubMed]
- Dangi-Garimella S, Sahai V, Ebine K, et al. Three-dimensional collagen I promotes gemcitabine resistance in vitro in pancreatic cancer cells through HMGA2-dependent histone acetyltransferase expression. PLoS One 2013;8:e64566. [Crossref] [PubMed]
- Grzesiak JJ, Ho JC, Moossa AR, et al. The integrin-extracellular matrix axis in pancreatic cancer. Pancreas 2007;35:293-301. [Crossref] [PubMed]
- Öhlund D, Franklin O, Lundberg E, et al. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer 2013;13:154. [Crossref] [PubMed]
- Michl P, Gress TM. Improving drug delivery to pancreatic cancer: breaching the stromal fortress by targeting hyaluronic acid. Gut 2012;61:1377-9. [Crossref] [PubMed]
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418-29. [Crossref] [PubMed]
- Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013;62:112-20. [Crossref] [PubMed]
- Hingorani SR, Harris WP, Beck JT, et al. Exploratory biomarker results from early investigation of PEGPH20 in combination with gemcitabine (Gem) in patients with pancreatic cancer (PDA). J Clin Oncol 2015;33:abstr 300.
- Hingorani SR, Harris WP, Beck JT, et al. Final results of a phase Ib study of gemcitabine plus PEGPH20 in patients with stage IV previously untreated pancreatic cancer. J Clin Oncol 2015;33:abstr 359.
- Hingorani SR, Harris WP, Beck JT, et al. A phase Ib study of gemcitabine plus PEGPH20 (pegylated recombinant human hyaluronidase) in patients with stage IV previously untreated pancreatic cancer. J Clin Oncol 2013;31:abstr 4010.
- Hingorani SR, Harris WP, Hendifar AE, et al. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: Interim results of a randomized phase II study. J Clin Oncol 2015;33:abstr 4006.
- Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612-6. [Crossref] [PubMed]
- Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res 2013;19:6286-95. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007;13:2151-7. [Crossref] [PubMed]
- Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828-33. [Crossref] [PubMed]
- McAllister F, Bailey JM, Alsina J, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 2014;25:621-37. [Crossref] [PubMed]
- Soares KC, Rucki AA, Wu AA, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 2015;38:1-11. [Crossref] [PubMed]
- Middleton G, Silcocks P, Cox T, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol 2014;15:829-40. [Crossref] [PubMed]
- Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33:1325-33. [Crossref] [PubMed]
- Erkan M, Michalski CW, Rieder S, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 2008;6:1155-61. [Crossref] [PubMed]
- Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 2004;29:179-87. [Crossref] [PubMed]
- Sinn M, Denkert C, Striefler JK, et al. α-Smooth muscle actin expression and desmoplastic stromal reaction in pancreatic cancer: results from the CONKO-001 study. Br J Cancer 2014;111:1917-23. [Crossref] [PubMed]
- Infinity Pharmaceuticals. Infinity Reports Update from Phase 2 Study of Saridegib Plus Gemcitabine in Patients with Metastatic Pancreatic Cancer. Available online: http://www.reuters.com/article/idUS101395+27-Jan-2012+BW20120127
- Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol 2015;12:319-34. [Crossref] [PubMed]
- Bijlsma MF, van Laarhoven HW. The conflicting roles of tumor stroma in pancreatic cancer and their contribution to the failure of clinical trials: a systematic review and critical appraisal. Cancer Metastasis Rev 2015;34:97-114. [Crossref] [PubMed]
- Froeling FE, Feig C, Chelala C, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology 2011;141:1486-97, 1497.e1-14.
- Xu Z, Pothula SP, Wilson JS, et al. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol 2014;20:11216-29. [Crossref] [PubMed]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004;4:528-39. [Crossref] [PubMed]
- Gore J, Korc M. Pancreatic cancer stroma: friend or foe? Cancer Cell 2014;25:711-2. [Crossref] [PubMed]
- Elgueta R, Benson MJ, de Vries VC, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009;229:152-72. [Crossref] [PubMed]
- Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med 1999;5:780-7. [Crossref] [PubMed]
- Sica A, Larghi P, Mancino A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol 2008;18:349-55. [Crossref] [PubMed]
- Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res 2008;14:1455-63. [Crossref] [PubMed]
- Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168-78. [Crossref] [PubMed]


