The clinical utility of normal range carbohydrate antigen 19-9 level as a surrogate marker in evaluating response to treatment in pancreatic cancer—a report of two cases
Introduction
Carbohydrate antigen 19-9 (CA 19-9) was isolated and reported by Koprowski et al. for the first time in 1979 (1). Even though it was not widely used clinically (2), it was the only available serum biomarker for pancreatic cancer (PC) (3). Previous report suggested the usefulness of serial monitoring of CA 19-9 levels in patients with resected PC post curative surgery (4). Serial monitoring of CA 19-9 levels is also useful for patients who receive chemotherapy for advanced disease (4). Several different groups have demonstrated a correlation between longevity of patients receiving chemotherapy for advanced disease and the level of CA 19-9 (5-10). These studies suggest a declining level of CA 19-9 in those patients receiving chemotherapy. In addition, it is also observed a prognostic impact of CA 19-9 in those patients (2). Considering this, the American Society of Clinical Oncology (ASCO) recommended Ca 19-9 measurement prior to any PC treatment. ASCO also recommended serial measurements of CA 19-9 during the therapy of advanced PC (once in every 1 to 3 months) (11).
After a decade from Koprowski and coworkers reporting the usefulness of CA 19-9, it has become the most widely used blood test among PC patients. It has a higher sensitivity (80% approx.) and specificity (90% approx.) with an upper limit of 37 U/mL cut-off value (12). CA 19-9 is also widely used in predicting unresectability of pancreatic adenocarcinoma (12). It has been reported that, at least 96% of tumors that result in blood levels greater than 1,000 U/mL have been found to be unresectable (3).
In it is widely accepted that CA 19-9 level is only clinically useful if it was elevated above the normal range. In this report, we report two cases were normal range CA 19-9 level has been found to be a useful tool (surrogate marker) in following PC activity and in response to treatment in patients with PC.
Case presentation
Case 1
A 56-year-old Caucasian female with a localized PC confined to the neck of the pancreas, went under a pancreaticoduodenectomy with a positive margin. The surgical pathology was reported with concerning microscopic positive margin at the proximal and distal portal vein margins and had a pathological staging of pT3N1Mx adenocarcinoma of the pancreas according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, Seventh Edition [2010]. pT3 defined as tumor extends beyond the pancrease but without the involvement of the celiac axis or the superior mesentric artery, pN1 defined as regional lymph nodes metastasis, the patient had 2 out of 30 lymph nodes involved, Mx distance metastasis can not be assessed, clinically the patient had M0 which is defined as no evidence of distant metastasis.
The patient preoperatively had a normal range of CA 19-9 with a level of 6.5 U/mL (normal range, 0.0–35.0). However, we observed a post-surgery marginal decline to 5 U/mL (Table 1and Figure 1, A). At 8 weeks post-operative period, CT imaging shows no interval development of metastatic disease. She underwent adjuvant systemic chemotherapy (gemcitabine 600 mg/m2 for 3 weeks on and 1 week off schedule for 4 cycles) followed by radiation treatment with stable CA 19-9 value of 7.1 U/mL (Table 1 and Figure 1, B). An increase in the CA 19-9 level to (9.8 U/mL) (Table 1 andFigure 1, C) was noted during surveillance, this was consistent with the imaging studies in keeping with evidence of metastatic disease with peritoneal implant. The patient received 6 cycles of systemic palliative chemotherapy (6 cycles of GAX, each cycle is administered every 2 weeks, Gemcitabine at 400 mg/m2, Abraxane (nab-paclitaxel) at 100 mg/m2 and Xeloda (capecitabine) at 1,350 mg/m2 which lead to marginal decline in the CA 19-9 level to 8.5 U/mL (Table 1 and Figure 1, D).

Full table
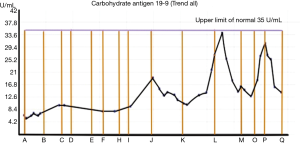
The patient had a response to the treatment based on imaging studies which was associated with a decline in CA 19-9 level to 8 U/mL (Table 1 and Figure 1, E). The CA 19-9 level plateaued (7.8 U/mL), without any sign of active disease on imaging studies surveillance continued (Table 1 and Figure 1, F-H). Gradually CA 19-9 level increased to 9.5 U/mL from the plateau level without any obvious clinical sign of progressive disease or as evidenced by imaging studies. Subsequently multifocal peritoneal implants was noted on imaging studies with a sharp increase in CA 19-9 value to 19.1 U/mL (Table 1 and Figure 1, J). After re-introducing of the systemic chemotherapy (GAX) for 6 cycles (similar dosing and schedule as above) this was followed by a decline of CA 19-9, from 19.1 to 10.5 U/mL and there was a radiological response. After 6 months from the last chemotherapy treatment, the CA 19-9 level had increased up to 26.8 U/mL which is still within the normal range yet this was associated with evidence of relapsed disease in the peritoneum, systemic chemotherapy (GAX) was reintroduced for a total of 6 cycles (similar dosing and schedule as above) with subsequent decline in the level to 16.2 U/mL (Table 1and Figure 1, M) in keeping with response to the systemic treatment as confirmed by the imaging studies.
The serum CA 19-9 level declined for few months then peaked up to 16 U/mL without any sign of progression of disease as evidenced from the imaging studies (Table 1and Figure 1, O). However, the serum CA 19-9 level continued to rise and peaked to 30 U/mL with evidence of recurrent peritoneal disease on imaging CT (Table 1 and Figure 1, P). Systemic chemotherapy (GAX) was reintroduced for a total of 2 cycles (similar dosing and schedule as above). After re-introducing the systemic chemotherapy the CA 19-9 level declined to 14 U/mL (Table 1 and Figure 1, Q). Afterwards we noted a sharp peak in the CA 19-9 level which reached to 40 U/mL and which is above the upper limit of normal for CA 19-9 as per our institution (normal, 0.0–35.0). Imaging studies confirmed the progression of disease and patient currently receiving GAX systemic chemotherapy (similar dosing and schedule as above).
Case 2
The second case we are reporting a 68-year-old Caucasian female with borderline resectable pancreatic head adenocarcinoma. The preoperative CA 19-9 level of the patient was 12.5 U/mL (normal range, 0.0–35.0). The patient received neoadjuvant chemotherapy as part of a clinical trial with gemcitabine/erlotinib (gemcitabine 600 mg/m2 for 3 weeks on and 1 week off schedule for 6 cycles and erlotinib orally at 100 mg daily for 6 months (Table 2 and Figure 2, A).

Full table
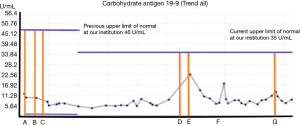
A post-surgery decline of the CA 19-9 level to 10 U/mL was observed (Table 2 and Figure 2, B) the patient underwent pancreaticoduodenectomy with negative margin—final pathological staging pT3N1MX adenocarcinoma of the pancreases according to the AJCC Cancer Staging Manual, Seventh Edition [2010]. pT3 defined as tumor extends beyond the pancreas but without the involvement of the celiac axis or the superior mesenteric artery, pN1 defined as regional lymph nodes metastasis, the patient had 5 out of 34 lymph nodes involved, Mx distant metastasis cannot be assessed, clinically the patient had M0 which is defined as no evidence of distant metastasis.
After the surgery the patient underwent adjuvant gemcitabine chemotherapy and had a marginal decline in the CA 19-9 to 9 U/mL (Table 2and Figure 2, C).
On subsequent surveillance, the patient was noted to have an increase in the CA 19-9 level to 16 U/mL (Table 2 and Figure 2, D) imaging studies did not demonstrate any evidence of disease, patient continued surveillance, CA 19-9 level further increased to 22 U/mL (Table 2 and Figure 2, E). This was associated with peritoneal metastasis in the imaging studies; palliative systemic chemotherapy (GAX) was introduced for metastatic disease at similar dosing and schedule as case 1. Subsequently the CA 19-9 level decreased in keeping with response to systemic treatment which was confirmed on imaging studies. Recurrent peritoneal metastasis disease responded to re-introduction of palliative chemotherapy (4 cycles of GAX).
Unfortunately the patient experienced vaginal local recurrence with the CA 19-9 level increase to 13 U/mL, this was treated with concurrent chemoradiation with capecitabine and CA 19-9 declined to 6.5 U/mL from an initial value of (Table 2 and Figure 2, G). The patient is currently under surveillance without any evidence of active disease.
Discussion
Patients with PC mostly require multimodalities treatment including surgery, radiation and chemotherapy either in the neoadjuvant, adjuvant or metastatic settings (5). Multiple studies have demonstrated that a treatment related decline in CA 19-9 serum levels is associated with prolonged survival and is an independent predictor of overall survival (5,10,13,14). CA 19-9 has been used extensively in the clinical settings to assess response to treatment for PC (10). In clinical research, CA 19-9 has also been used as a surrogate marker in neoadjuvant, adjuvant and also metastatic disease (15,16).
Several previously reported studies have explored the utility and sensitivity of CA 19-9 serum levels as a screening tool for PC (12,17-19). Previous reporters suggested an optimal value of CA 19-9 in serum as >37 U/mL (20), although the sensitivity and specificity were in a range of 96% to 99% respectively. CA 19-9 levels also have low specificity (20). CA 19-9 is frequently elevated in patients with cancers other than PC and various benign pancreaticobiliary disorders (12,21-24). One study found that serum concentrations above 37 U/mL represented the most accurate cut-off value for discriminating PC from benign pancreatic disease, but the sensitivity and specificity for PC at this level were only 77 and 87 percent, respectively (25). Furthermore, the positive predictive value (PPV) is low, particularly among asymptomatic individuals. In a large series of over 70,000 asymptomatic individuals, the PPV of a serum CA 19-9 level >37 U/mL was only 0.9% (26). Because of this, expert guidelines recommend against the use of CA 19-9 as a screening test for PC (11). Even among symptomatic individuals (epigastric pain, weight loss, jaundice), the sensitivity, specificity, and PPV of an elevated CA 19-9 >37 U/mL level are only approximately 80%, 85%, and 72% (3,12).
Clinically CA 19-9 level has only been serially measured if the initial level has been elevated on presentation. Most clinicians usually stop measuring the CA 19-9 serum level if the level is within normal range. The CA 19-9 level in previously reported cases have been within the normal range. Despite that, the CA 19-9 level has been a useful surrogate marker to follow the patients’ progression and response to treatment in neoadjuvant, adjuvant and metastatic settings. Both patients had a resectable PC, an indolent course of disease and sensitivity to systemic chemotherapy treatment with decline in the CA 19-9 associated with imaging response and clinical improvement.
In one report at least 76.9% of stage III PC patients with a CA 19-9 serum level of <37 U/mL survived more than 5 years (27). In another retrospective study concluded that an elevated preoperative CA 19-9 serum levels of >200 U/mL, a high-grade tumor, an R2 resection independently predicted early death (20). In another study, 129 subjects with surgically resected PC were grouped based on their pre-operative CA 19-9 level [undetectable, normal (<37 U/mL), 38–200 U/mL, and >200 U/mL]. It was found that patients with undetectable pre-operative CA 19-9 serum levels and serum levels of <37 U/mL had an improved survival rate compared to patients with CA 19-9 serum levels between 37–200 U/mL (28). These studies give an insight into various cut-off levels for pre-operative CA 19-9 serum levels in an effort to predict survival among PC patients.
CA 19-9 requires the presence of the Lewis blood group antigen (a glycosyltransferase) to be expressed. Among individuals with a Lewis-negative phenotype (an estimated 5 to 10 percent of the population) the CA 19-9 is normal (16,29). The concentration of the tumor marker CA 19-9 is influenced by the patient’s secretor status and Lewis genotype, which is determined by the immunological reactivities with Lewis carbohydrate antigens on blood cells (29). It is also known that genetic background of the patient influence the overall Lewis structures on the cell membrane (16). The structure A1 Lewis b is more complex than the structure Lewis b, which creates a discrepancy between Lewis positive and Lewis negative individuals (29). In addition to the Lewis genotype, CA 19-9 concentrations in secretor individuals normally depend on the Secretor genotypes (Se/Se or Se/se) (29). Patients with heterozygous mutated in the Secretor gene shows higher concentrations of CA 19-9 in serum than individuals who are homozygous wild type (29).
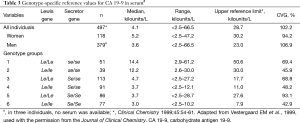
As mentioned above, a study by Vestergaard et al., Reported the mean level of CA 19-9 for subgroups with different secretor and Lewis genotypes were found to be 28.7 kilounits/L (29). However, for secretors and nonsecretors groups, the upper limits were detected as 12.4 and 61.2 kilounits/L, respectively (29) (Table 3). This partitioning of the data was originally described by another group (30), where it was reported that a statistical difference between the different subgroups represents one possible way to increase the clinical utility of CA 19-9 tumor marker.
Full table
In our two patients, we were unable to determine the secretory and Lewis genotype as the test was not available in our institution, we suspect the secretory status of the patients was contributing to the low level of CA 19-9, as evident in the first patient, The CA 19-9 level has increased above the upper limit of normal as the tumor became more aggressive (based on imaging studies) and more resistant to chemotherapy which indicates likely the patient has positive Lewis genotype.
Conclusions
CA 19-9 is the most extensively studied and widely used biomarkers for treating PC. CA 19-9 is usually not serially measured if the initial value is within normal range. However, from our two reported cases we suggest that measuring and following serum CA 19-9 in patient with normal range values may be helpful in monitoring a subset of this population who may be undersecretors, this may help in following PC disease activity in response to various treatment modalities specially in patients with low volume of disease which can be difficult to assess response on imaging studies only.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Koprowski H, Herlyn M, Steplewski Z, et al. Specific antigen in serum of patients with colon carcinoma. Science 1981;212:53-5. [Crossref] [PubMed]
- Boeck S, Stieber P, Holdenrieder S, et al. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology 2006;70:255-64. [Crossref] [PubMed]
- Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol 2007;33:266-70. [Crossref] [PubMed]
- Safi F, Schlosser W, Kolb G, et al. Diagnostic value of CA 19-9 in patients with pancreatic cancer and nonspecific gastrointestinal symptoms. J Gastrointest Surg 1997;1:106-12. [Crossref] [PubMed]
- Halm U, Schumann T, Schiefke I, et al. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer 2000;82:1013-6. [PubMed]
- Ko AH, Hwang J, Venook AP, et al. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer 2005;93:195-9. [Crossref] [PubMed]
- Heinemann V, Schermuly MM, Stieber P. CA19-9: a pedictor of response in pancreatic cancer treated with gemcitabine and cisplatin. Anticancer Res 1999;19:2433-5. [PubMed]
- Ziske C, Schlie C, Gorschlüter M, et al. Prognostic value of CA 19-9 levels in patients with inoperable adenocarcinoma of the pancreas treated with gemcitabine. Br J Cancer 2003;89:1413-7. [Crossref] [PubMed]
- Bauer TM, El-Rayes BF, Li X, et al. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer 2013;119:285-92. [Crossref] [PubMed]
- Reni M, Cereda S, Balzano G, et al. Carbohydrate antigen 19-9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer 2009;115:2630-9. [Crossref] [PubMed]
- Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 2006;24:5313-27. [Crossref] [PubMed]
- Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol 1990;85:350-5. [PubMed]
- Saad ED, Machado MC, Wajsbrot D, et al. Pretreatment CA 19-9 level as a prognostic factor in patients with advanced pancreatic cancer treated with gemcitabine. Int J Gastrointest Cancer 2002;32:35-41. [Crossref] [PubMed]
- Maisey NR, Norman AR, Hill A, et al. CA19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br J Cancer 2005;93:740-3. [Crossref] [PubMed]
- Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 2012;3:105-19. [PubMed]
- Koom WS, Seong J, Kim YB, et al. CA 19-9 as a predictor for response and survival in advanced pancreatic cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2009;73:1148-54. [Crossref] [PubMed]
- Kim JE, Lee KT, Lee JK, et al. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol 2004;19:182-6. [Crossref] [PubMed]
- Satake K, Takeuchi T, Homma T, et al. CA19-9 as a screening and diagnostic tool in symptomatic patients: the Japanese experience. Pancreas 1994;9:703-6. [Crossref] [PubMed]
- Chang CY, Huang SP, Chiu HM, et al. Low efficacy of serum levels of CA 19-9 in prediction of malignant diseases in asymptomatic population in Taiwan. Hepatogastroenterology 2006;53:1-4. [PubMed]
- Waraya M, Yamashita K, Katagiri H, et al. Preoperative serum CA19-9 and dissected peripancreatic tissue margin as determiners of long-term survival in pancreatic cancer. Ann Surg Oncol 2009;16:1231-40. [Crossref] [PubMed]
- DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology 1999;117:1464-84. [Crossref] [PubMed]
- Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol 1999;10 Suppl 4:145-9. [Crossref] [PubMed]
- Kim HJ, Kim MH, Myung SJ, et al. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: analysis using a receiver operating characteristic curve. Am J Gastroenterol 1999;94:1941-6. [Crossref] [PubMed]
- Molina V, Visa L, Conill C, et al. CA 19-9 in pancreatic cancer: retrospective evaluation of patients with suspicion of pancreatic cancer. Tumour Biol 2012;33:799-807. [Crossref] [PubMed]
- Cwik G, Wallner G, Skoczylas T, et al. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg 2006;141:968-73. [Crossref] [PubMed]
- Paganuzzi M, Onetto M, Marroni P, et al. CA 19-9 and CA 50 in benign and malignant pancreatic and biliary diseases. Cancer 1988;61:2100-8. [Crossref] [PubMed]
- Smith RA, Bosonnet L, Ghaneh P, et al. Preoperative CA19-9 levels and lymph node ratio are independent predictors of survival in patients with resected pancreatic ductal adenocarcinoma. Dig Surg 2008;25:226-32. [Crossref] [PubMed]
- Berger AC, Meszoely IM, Ross EA, et al. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol 2004;11:644-9. [Crossref] [PubMed]
- Vestergaard EM, Hein HO, Meyer H, et al. Reference values and biological variation for tumor marker CA 19-9 in serum for different Lewis and secretor genotypes and evaluation of secretor and Lewis genotyping in a Caucasian population. Clin Chem 1999;45:54-61. [PubMed]
- Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference ranges. Clin Chem 1990;36:265-70. [PubMed]


