Ki67 score as a potential predictor in the selection of liver-directed therapies for metastatic neuroendocrine tumors: a single institutional experience
Introduction
Neuroendocrine tumors (NET) are relatively slow-growing tumors that arise commonly from the gastrointestinal and pulmonary systems. The most common types include carcinoids, islet cell tumors, paragangliomas, pheochromocytomas and medullary thyroid cancers. Considered rare, their incidence has increased 6.3% in the last 25 years with a prevalence of 35 per 100,000 patients (1,2). Due to their indolent nature, patients present with a variety of vague symptoms, which often leads to a delay in diagnosis by 5–7 years. As a result, between 46–93% of patients present with synchronous liver metastasis and another 25% develop metachronous disease during the intervening period (3,4). Even though these tumors are believed to have a more indolent biology, the presence of liver metastasis decreases 5-year survival rates from 75–99% to 20–40% (2,3,5). Indeed, approximately 80% of patients with hepatic metastasis at the time of diagnosis die within 5 years (3,5). Because of these factors, the treatment of metastatic lesions to the liver has become a major component of the overall treatment planning in these patients.
Despite its significance, the optimal treatment for metastatic hepatic lesions has remained controversial. This is, in part, due to multiple factors including overall health, burden of the disease, liver function, presence or absence of extra hepatic disease, and access to technology that can reduce the disease burden. Surgical resection is preferred if greater than 90% of the disease can be safely removed. It has been shown to be superior to other therapies, especially with regards to symptomatic relief, time to disease progression, and better 5-year survival rate (6-9). However, only 5–15% of patients with liver metastases are surgical candidates and nearly 50% recur after resection (10,11). Therefore, in these cases, alternate strategies such as liver directed ablation, peptide receptor radionuclide therapy, transarterial embolization (TAE), transarterial chemoembolization (TACE) or transarterial radioembolization (TARE) with yttrium-90 (Y-90) have been employed to reduce tumor burden.
TAE entails using beads to block the tumor microvasculature, which results in ischemic damage and necrosis. The addition of chemotherapy to TAE, referred as TACE, with or without drug eluting beads (DEB), has been suggested to further improve outcomes in some studies (12). More recently, TARE has emerged as a well-tolerated embolization technique (13,14). This method uses resin or glass microspheres (20–30 μm) loaded with Y-90, a radioisotope that emits pure beta radiation. The main advantages of Y-90 are a short half-life of 64 hours and a low mean penetrance depth of 2–3 mm, which allows the radiation to be contained within the tumor bed with relative sparing of the surrounding parenchyma (15). There is tumor damage by both ischemia as well as radiation. While both Y-90 & TACE have been shown to prolong survival (12-14), the data confirming the superiority of one embolization technique over the other has been conflicting. As a result, the criteria for patient selection for one therapy over the other are lacking and have remained unclear.
The Ki-67 index is a scoring system that measures proliferation and growth of cells (16). It has been shown to predict biological behavior, response to chemotherapy, and survival of patients with different types of tumors, including NETs (16-18). It was thus hypothesized that the Ki-67 proliferative index can predict the optimal treatment, and therefore our team sought to determine if the Ki67 score could be utilized to select the optimal treatment strategy in these cases.
Methods
The Institutional Review Board at Roswell Park Cancer Institute approved this study. This study included 72 patients with metastatic NETs who were treated at our institution with liver directed Y-90 or TACE between 2001 and 2014. The treatment modality chosen was based upon physician preference (after multi-disciplinary discussions) and insurance coverage; no definitive criteria were available to prefer one treatment modality over the other. All these patients had biopsy confirmed NETs. A pathologist who was blinded to the clinical data completed the Ki67 staining in 44 patients. A Ki67 score < or ≥3 was selected to allow for separation of patients into groups based on low grade versus intermediate and high grade characteristics. The primary end-point of this study was death from any cause. Patients were evaluated from the date of their first treatment to either the date of last clinical follow-up or death.
Immunohistochemical analysis
Paraffin-embedded tumors were cut into 4-μm thick sections and placed on coated glass slides. The sections were de-waxed in xylene and rehydrated in a series of graded ethanol solutions, and the endogenous peroxidase activity was blocked with a 0.3% H2O2 methanol solution. Before application of the primary antibody, the slides were subjected to antigen retrieval by heating in 10 mM citrate buffer (pH 6.0) for 15 min at 121 °C in an autoclave oven. Then, MIB-1 monoclonal antibodies (Ki67) were applied, and slides were incubated overnight at 4 °C. The slides were rinsed in 0.01 mol/L phosphate-buffered saline, and bound antibodies were detected with the simple stain MAX PO (R) using 3,3'-diaminobenzidine tetrahydrochloride as the substrate. The peroxidase reaction was visualized with 0.02% 3,3'-diaminobenzidine tetrahydrochloride containing 0.005% H2O2 in 0.01 M tris-phosphate buffer (pH 7.4). Finally, the sections were counterstained with hematoxylin. A single pathologist calculated the Ki67 score individually.
Statistical analysis
Patient demographics, clinical variables and treatment characteristics were analyzed as groups using the mean, median, standard deviation and ranges. Frequencies and relative frequencies were utilized for categorical variables. Comparisons were made between groups using the Mann-Whitney U and Fisher’s exact tests. Overall survival was summarized using standard Kaplan-Meier methods, with comparisons made using the log-rank test. Multivariate analysis of survival data utilized Cox regression models; where, in addition to treatment status & Ki67 score, variables were included using the backward selection method (alpha-exit =0.05). The models were fit using Firth’s penalized function and hazard ratios, with corresponding confidence intervals obtained from the model estimates. All analyses were two-sided and conducted in SAS v9.4 (Cary, NC, USA) at a significance level of 0.05.
Results
Patient characteristics
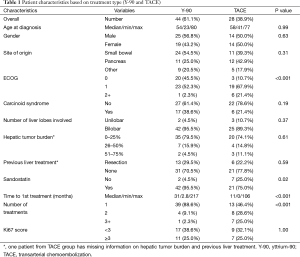
During the study period between 2001 and 2014, a total of 84 patients were available for analyses. Of these, 12 patients were excluded from the analyses due to inadequate post-treatment follow-up (n=7) or incomplete documentation (n=5). Seventy-two patients who underwent 100 treatments were included in the final analysis. There were 39 males (54.2%) and 33 females (45.8%). The median age of patients was 57 years (range, 23–80), and the median disease follow-up period was 54.5 months (range, 1–143.8 months). The most common sites of origin for primary NETs were small bowel (n=35, 48.6%) and pancreas (n=23, 31.9%). Majority of patients had performance status ECOG score 0 or 1 (n=65, 90.2%) and nearly all patients (n=63, 87.5%) received octreotide therapy prior to liver directed treatment.
With regards to the metastatic spread of the NETs, bilobar hepatic distribution was seen in 67 patients (93.1%) and extra-hepatic spread was observed in 52 patients (72.2%). Nineteen patients (26.8%) were noted to have previous history of hepatic resection prior to consideration for liver directed therapy.
Treatment characteristics
Amongst 72 patients treated with liver directed therapy, 44 patients underwent radio-embolization with Y-90 and 28 patients received TACE (Table 1). While the number of patients were proportionally higher in the Y-90 group compared to the TACE group (61.1% and 38.9%, respectively), the number of total procedures were similar in both groups (n=50, respectively). Comparing patient demographics, we observed no significant statistical difference with regards to distribution of age (P=0.995), gender (P=0.632), primary site of tumor origin (P=0.305) or presence of carcinoid syndrome symptoms (P=0.195) between the groups. Further, patients in the Y-90 group had higher incidence of carcinoid syndrome (38.6% vs. 21.4%, P=0.195) and bilobar liver disease (95.5% vs. 89.3%, P=0.371), whereas hepatic tumor burden >25% was greater in the TACE group (25.9% vs. 20.4%, P=0.614), but these differences were also not significant.
Full table
On comparing treatment groups with respect to ECOG performance score (P=0.001), concomitant use of Sandostatin (P=0.023), time (in months) from initial diagnosis until first treatment (P<0.001), and the number of the specified treatments completed (P<0.001), we did find statistically significant differences. The need for repeated treatments to achieve similar results (53.6% vs. 11.4%) was higher in TACE group, while patients in the Y-90 group had a higher percentage of concomitant use of Sandostatin (95.5% vs. 75%) and time from diagnosis to first treatment compared to TACE (median, 31.6 and 10.7 months, respectively).
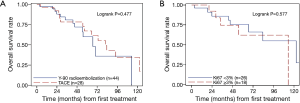
No significant difference in overall survival was noted between patients treated with Y-90 versus TACE. The median survival in the Y-90 and TACE groups were 66.8 months (range, 54.4–113.1) and 81.9 months (range, 54.8–122.6), and the corresponding 5-year survival rates were 0.60 (95% CI, 0.4–0.8) and 0.67 (95% CI, 0.4–0.8), respectively (P=0.477) (Figure 1).
Association between treatment groups with Ki67 score
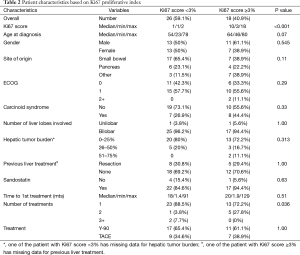
The Ki67 prognostic index score was available in 44 patients (61.1%). Table 2 lists patient characteristics for all patients with corresponding Ki-67 scores (<3 and ≥3). Of note, none of the patients in our cohort had Ki-67 scores >20% (high grade or poorly differentiated tumors). On univariate analysis, all variables except number of treatments required to achieve stable response were statistically non-significant. Patients with Ki-67 score <3 were most likely to benefit using a single procedure (including both the right and the left side) whereas patients with Ki67 ≥3 required more than one procedure (P=0.036).
Full table
Clinical outcomes
On analyzing individual variables, using the log rank, it was noted that the overall survival was not statistically dependent on the treatment type (Figure 1A; P=0.477) or Ki67 score (Figure 1B; P=0.577).
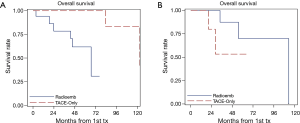
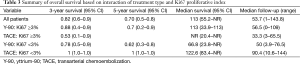
However, when OS was modeled as a function of both treatment method and Ki-67 expression and examined using the Cox regression, there was a significant main effect of Ki67 (P=0.029) and interaction (P=0.0274) with treatment type. The significant interaction indicates that the effect of treatment is dependent on the level of Ki67 score. The Kaplan-Meier curves illustrate these interactions in overall survival based on type of treatment and Ki67 score (Figure 2A,B). The hazard ratio obtained for treatment type (Y-90 versus TACE) in patients with Ki67 score <3 is 10.6 (95% CI: 1.1–101.0). This indicates that patients who underwent TACE have better survival outcomes as compared to the Y-90 patients. The converse is true in the higher Ki67 (≥3) group, where the hazard ratio for treatment type is 0.3 (95% CI, 0.03–1.9). While not statistically significant, it is a strong indicator towards improved survival outcomes in Y-90 patients. The improvement in survival is also reflected at 3-year, and 5-year survival rates, respectively (Table 3).
Full table
The multivariate analysis, with disease site (P=0.042) as the only additional variable retained through backwards selection, further supports these results (Table 4). There was a significant main effect for treatment type (P=0.034) and Ki67 (P=0.031), and interaction (P=0.008). The hazard ratios corresponding to treatment type are 13.5 (95% CI, 1.2–148.9) and 0.1 (95% CI, 0.01–0.9) for the low and high Ki67 patients respectively (Table 4).

Full table
Discussion
The development of an ideal treatment algorithm for NET liver metastases remains a clinical challenge. One of the biggest obstacles in developing a pathway is determining what treatment modality will most benefit patients who have unresectable disease. A number of factors have been identified that may impact survival in these patients. A recent study by Ahmed et al showed that increasing levels of plasma chromogranin-A, high Ki67 score, high tumor volume and treatment with chemotherapy are associated with poor outcome, while resection of liver metastases, resection of small bowel primary, and treatment with octreotide are associated with improved prognosis. In addition, the Ki67 level and resection of the primary lesion act as independent predictors of survival (19).
With regards to treatment of metastatic lesions, the use of aggressive liver-directed therapy has been shown to improve survival (5,8,20). Similar to these studies, the OS was prolonged in our cohort, but there was no significant difference in OS when patients were compared on basis of treatment type alone (Y-90 versus TACE; median survival, 69 versus 82 months). This by itself was indicative that neither of these liver directed therapies was superior to the other in terms of survival benefit when used in a non-directed manner. This finding is not surprising, as previous studies have shown that both Y-90 and TACE are viable options for the liver-directed therapy of metastases and little data exists to validate one therapy over the other for prolongation of OS. Due to the lack of prospective randomized trials, there is persistent controversy regarding the therapy that benefits the most.
Another aspect of our study was the finding that the Ki-67 proliferative index scores alone did not have any predictive influence on our patient cohort. The Ki-67 score cutoff value (<3 and ≥3) was chosen based on the assumption that the biology of low grade tumors versus intermediate/high grade tumors would be different and help in predicting survival benefits. The OS did not change when patients were stratified by our Ki-67 score cutoff regardless of treatment method and thus, no statistically significant difference in overall survival was observed (Figure 1B). However, when OS was molded as a function of both treatment method and Ki-67 expression and examined using Cox regression and multivariate analysis, we noted statistically significant improvement in HR when Y-90 was utilized for treating patients with Ki-67 score ≥3 and vice-versa when TACE was used for patients with Ki-67 score <3 (Table 4). This interaction between treatment type and Ki67 score seems to correlate with ‘tumor biology’, implying a possibility that tumors with aggressive histology (intermediate grade; Ki67 score ≥3) respond better to radiation and less aggressive (low grade; Ki67 score <3) fair better with TACE. Further, since Cox regression analysis (using the hazard ratios) is assumed to be better at detecting relationships, this would explain why the initial results using the log rank test would appear to be non-significant. This also represents a more accurate picture of the data than Kaplan Meier curves alone.
On evaluating the 3-year and 5-year survival rates (Table 4), we see a survival difference that is not statistically significant. This is either a likely result of a relatively small sample size that did not have enough power to detect this difference in our patient cohort or the result of utilization of other systemic treatments for some of our patients including octreotide or tyrosine kinase inhibitors that have been shown to prolong survival. This could also be partially related to selection bias where in the past we treated military disease with Y-90 and larger or fewer number of tumors with TACE. While this may be more accurately discerned in a larger prospective study with longer follow-up, based on our analysis and our current understanding of the Ki-67 score and these treatment types, we believe that our data justifiably reflects the relation between tumor biology (low grade versus intermediate grade) and the physiological concept of treatment with Y-90 and TACE. Furthermore, Ki-67 is also a very subjective measure and we tried to eliminate possible bias from this by having a single pathologist analyze the Ki-67 score using consistent controls.
In summary, there is a significant interaction between the Ki-67 score and the type of liver directed therapy utilized for hepatic metastasis in patients with NETs. In patients with Ki67 score ≥3, patients are more likely to achieve treatment benefit with Y-90 and for patients with Ki67 score <3, TACE appears more effective.
Acknowledgements
This study was in part supported by an independent award granted by SirTex Medical, Inc.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg 2004;240:117-22. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Nazario J, Gupta S. Transarterial liver-directed therapies of neuroendocrine hepatic metastases. Semin Oncol 2010;37:118-26. [Crossref] [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [Crossref] [PubMed]
- Veenendaal LM, Borel Rinkes IH, Lips CJ, et al. Liver metastases of neuroendocrine tumours; early reduction of tumour load to improve life expectancy. World J Surg Oncol 2006;4:35. [Crossref] [PubMed]
- Hill JS, McPhee JT, McDade TP, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer 2009;115:741-51. [Crossref] [PubMed]
- Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg 1998;187:88-92; discussion 92-3. [Crossref] [PubMed]
- Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg 2005;241:776-83; discussion 783-5. [Crossref] [PubMed]
- Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin N Am 2003;12:231-42. [Crossref] [PubMed]
- Ihse I, Persson B, Tibblin S. Neuroendocrine metastases of the liver. World J Surg 1995;19:76-82. [Crossref] [PubMed]
- Sutcliffe R, Maguire D, Ramage J, et al. Management of neuroendocrine liver metastases. Am J Surg 2004;187:39-46. [Crossref] [PubMed]
- Ruutiainen AT, Soulen MC, Tuite CM, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol 2007;18:847-55. [Crossref] [PubMed]
- Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 2008;31:271-9. [Crossref] [PubMed]
- Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg 2008;247:1029-35. [Crossref] [PubMed]
- Ahmadzadehfar H, Biersack HJ, Ezziddin S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin Nucl Med 2010;40:105-21. [Crossref] [PubMed]
- Vilar E, Salazar R, Pérez-García J, et al. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer 2007;14:221-32. [Crossref] [PubMed]
- Palazzo M, Lombard-Bohas C, Cadiot G, et al. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumorr Eur J Gastroenterol Hepatol 2013;25:232-8. [Crossref] [PubMed]
- Nadler A, Cukier M, Rowsell C, et al. Ki-67 is a reliable pathological grading marker for neuroendocrine tumors. Virchows Arch 2013;462:501-5. [Crossref] [PubMed]
- Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer 2009;16:885-94. [Crossref] [PubMed]
- Glazer ES, Tseng JF, Al-Refaie W, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford) 2010;12:427-33. [Crossref] [PubMed]


