Small caliber covered self-expanding metal stents in the management of malignant dysphagia
Introduction
Self-expanding metal stents (SEMS) for management of malignant dysphagia were developed to address insertion and migration problems associated with legacy rigid non-expandable stents. The rigid stents typically had internal body diameters (BDs) of 10 to 14 mm and external BDs of up to 16 mm, which represented a compromise between the amount of dilation required for insertion and the degree of dysphagia relief to be achieved (1,2). Rigid stents with internal BDs of 14 mm resulted in significant dysphagia relief allowing most patients to be maintained on solid diets. However, placement of the rigid non-expandable stents resulted in acute procedure-related complication rates of 20% and mortality rates (MRs) of up to 16% (3).
In 1993, Knyrim et al. (4) conducted a pivotal randomized controlled trial of rigid stents with internal BDs of 12 mm versus SEMS with internal BDs of 15 mm. Patients randomized to rigid stents underwent preparatory balloon dilation to 20 mm. Patients randomized to SEMS were selectively dilated to 10 mm prior to stent placement. The median dysphagia score (DS) improved to 1 in both groups of patients. However, the rigid stent group experienced 14% procedure related mortality, whereas, the SEMS group experienced no procedure related mortality. Similar complication and MRs were observed by others (5-7). Uncovered SEMS rapidly replaced rigid stents for management of malignant dysphagia (8). However, frequent tumor in-growth obstruction problems were encountered (7,9-11). Covering the stent mesh reduced tumor in-growth, but increased SEMS migration rates (12-14). To address both the tumor in-growth problem and the migration problem, manufacturers pursued various design modifications to include covering only a portion of the SEMS body, increasing the SEMS BD, changing the shape of the SEMS body, or a combination of all three.
A randomized controlled trial by Siersema et al. (15) of partially covered and covered SEMS designs from three different manufacturers suggested that SEMS with BDs >20 mm were less likely to migrate when placed across distal esophagus and cardia malignant strictures. However, major stent related complications (perforation, bleeding, fever, pressure necrosis, severe pain) occurred in 18% to 36% of patients and overall 30-day mortality was 15%. In the multicenter randomized Dutch SIREC trial (16), 18 and 23 mm BD partially covered SEMS were compared to brachytherapy as palliation for inoperable esophageal adenocarcinoma. SEMS migration occurred in 17% and tumor in- or over-growth occurred in 15% of patients randomized to treatment with the partially covered SEMS. Major stent related complications occurred in 25% of patients. Stent related mortality was 9%.
The available data from existing studies suggests that the major complication and MRs for covered or partially covered SEMS with BDs ≥18 mm are similar to those produced by the non-expandable rigid legacy stents that SEMS were designed to replace. Upon review of the literature, we noted that the early studies of SEMS, which reported significant improvements in stent related complications and MRs, utilized SEMS with BDs of 15 or 16 mm (4,17). We postulated that the use of covered SEMS with BDs equal to or less than 16 mm for management of malignant dysphasia would minimize complication rates while providing adequate dysphagia control.
Methods
Study design and setting
After approval by our Institutional Review Board, informed consent was obtained for all endoscopic procedures and was specifically obtained for stent placement. The study aim was to determine if SEMS BD reduction produced a substantial decrease in stent related adverse event (AE) and MRs. The primary objectives of the study were to observe dysphagia response, stent migration rates, and AE and MRs, for small caliber covered SEMS (sccSEMS) with BDs between 10 and 16 mm when used in the management of malignant dysphagia. The secondary objectives of the study were to assess the technical success of direct endoscopic placement of sccSEMS (without fluoroscopy) and to assess the ease of removal of migrated SEMS. The study was designed as an observational cohort study (18).
Study patients
All patients with malignant dysphagia from either primary adenocarcinoma or squamous cell carcinoma (SCCA) of the esophagus were considered for enrollment. Patients with severe dysphagia and the following circumstances received sccSEMS: (I) patients unable to manage secretions without aspiration; (II) patients with incomplete response to neoadjuvant chemoradiation therapy awaiting surgery; (III) patients receiving chemotherapy with severe dysphagia not controlled by serial dilation; (IV) patients with terminal disease and severe dysphagia. Patients with dysphagia from external compression of the esophagus from non-esophageal cancer were excluded. Patients were enrolled over a 26-month period between January 1, 2008 and March 1, 2011. The clinical follow-up period extended to June 1, 2011.
After initial sccSEMS placement, patients returned at 6 to 8 week intervals for endoscopic surveillance. Endoscopy was also performed to investigate new symptoms and administer trans-stent therapies. Terminally ill hospice patients did not undergo surveillance endoscopy. Endoscopy was performed in asymptomatic patients when stent migration was noted incidentally on imaging studies. Patients were contacted by telephone within 24 to 48 hours after each endoscopy procedure and were also seen periodically in the Endosocpic Oncology Section’s outpatient clinics. Patients were followed until: (I) dysphagia resolved and stent therapy was no longer required as signified by anticipated stent migration; (II) death with stent in place; or (III) June 1, 2011.
Variables and data sources
DSs (19) were recorded as: 0 = no dysphagia; 1 = ability to eat some solid food; 2 = ability to eat some semisolid food only; 3 = ability to swallow liquids only; and 4 = inability to swallow saliva. DSs were assessed prior to sccSEMS placement, at 24 hour post procedure telephone follow-up, and at follow-up endoscopy. Procedure time for stent insertion was defined as the time from endoscope insertion to removal to include time for all interventions performed in addition to stent placement. Tumor location and measurements were obtained from endoscopy reports and discussion with the endoscopist when necessary. Stent migration events were classified as anticipated or unanticipated. Anticipated migration events occurred during local or systemic tumor therapy and were associated with an increase in stricture lumen diameter as estimated by the endoscopist at the time of stent extraction. Unanticipated migration events occurred in the absence of tumor therapy. Stent migration dates were calculated from the date of symptom onset or imaging study identification. Migration events were captured through patient symptom reports, the endoscopy surveillance process, and monitoring of imaging studies. AEs occurring immediately following sccSEMS placement were captured through use of a 24-hour post-endoscopy phone survey.
Small caliber stents
The Alimaxx-ES (Merit Medical Systems, Inc., South Jordan, UT, USA) esophageal stent became available in the fall of 2009. Prior to the Alimaxx-ES stent, a dedicated small caliber esophageal SEMS was not available in the United States. Therefore, small caliber fully covered biliary (Gore Viabil®, Conmed, Corp., Utica, NY, USA) and tracheobronchial (AERO DV®, Merit Medical Systems Inc., South Jordan, UT, USA) SEMS were used for purposes of this study during its initial phase (Table 1).
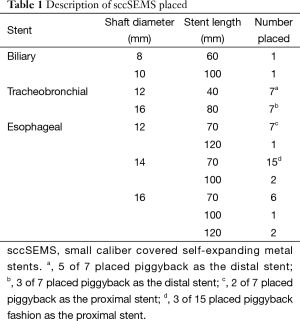
Full table
Stent insertion and removal techniques
Esophageal stent placement was performed on an outpatient basis. Patients received moderate to deep propofol sedation under the supervision of an anesthesiologist. The Olympus GIF Q180 videogastroscope (Olympus America, Inc., Center Valley, PA, USA) was used for initial endoscopic examination in all cases. If severe lumen compromise prohibited the passage of the GIF Q180 videogastroscope, the Olympus GIF XP 160 videogastroscope was used. All malignant strictures were traversed. Stricture length was measured and recorded. SEMS were selected such that they were 2 to 4 cm longer than the measured stricture.
After the malignant stricture was traversed, a flexible tip Savary guidewire (Cook Medical, Inc, Winston-Salem, NC, USA) was deployed and its tip positioned in the gastric antrum. Esophageal guidewire dilation was performed using Savary-Gillard® dilators (Cook Medical, Inc, Winston-Salem, NC, USA). The stricture lumen diameter was estimated based on the resistance encountered with either initial passage of the endoscope or the dilators. Graduated dilation was performed to increase the stricture lumen to 2 to 4 mm less than the desired stent BD.
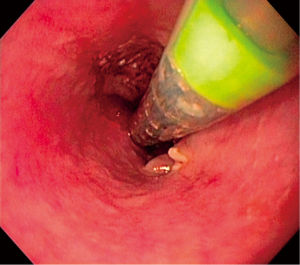
Upon completion of esophageal dilation, the sccSEMS introducer system was advanced over the guidewire and the videogastroscope was re-inserted adjacent to the sccSEMS introducer system. The proximal margin of the collapsed stent was positioned under direct vision such that it was 1 or 2 cm above the proximal margin of the malignant stricture (Figure 1). Stent deployment was then performed under direct endoscopic vision without the use of fluoroscopy. When appropriate length stents were unavailable, piggy back placement was used. After deployment, the videogastroscope passed into the stent to check for seating and positioning.
Patients received a trial of clear liquids in the recovery area. If no problems were encountered the patients were given dietary instructions specific to sccSEMS placement and discharged from recovery without further investigation.
When stent migration occurred, elective (20) outpatient removal was performed with the Olympus GIF Q180 videogastroscope and a rat tooth style forceps (Olympus Endotherapy, Center Valley, PA or Cook Medical, Inc, Winston-Salem, NC, USA). Edge grab and rotate maneuvers were typically used: (I) the proximal or distal stent edge was grasped such that it abutted the forceps jaw hinge; (II) the endoscope was rotated to achieve a view down the barrel of the stent; (III) the grasped stent was gently cinched up against the face of the endoscope; (IV) the instrument was withdrawn until the proximal edge of the stent engaged the distal margin of the stricture; (V) the endoscope was then gently rotated causing the sccSEMS to fold over on itself and easily clear the stricture with minimal trauma. Patients were not routinely subjected to barium radiography.
Statistical methods
Statistical analysis was performed by the Moffitt Cancer Center Biostatistics Department. Computations were conducted with SAS (Cary, NC, USA) program. The Wilcoxon Signed-Rank test was used to compare pre- and post-stent DSs. Anticipated and unanticipated stent migration rates and differences in migration rates between stent types were compared using Fisher’s Exact Test 2×2 Table Analysis. Kaplan-Meier product-limit analysis was performed on the time to event endpoints of interest. The effective duration for the initial stent placement (EDS1) was measured from the day of the initial sccSEMS’s insertion to the day of its migration, removal for any reason, or the patient’s death, whichever occurred first. The total effective durations of all stents placed in a given patient (EDSt) was defined as the time from the day of the initial sccSEMS’s insertion to the migration or removal for any reason of the last sccSEMS placed or the patient’s death, whichever occurred first. Both point estimates and 95% CI, when feasible and appropriate, were provided for important outcomes such as stent migration rates and median time-to-event endpoints (i.e., EDS1 and EDSt). A P value of <0.05 was considered statistically significant.
Results
Between January 1, 2008 and March 1, 2011, a total of 31 patients presenting to the endoscopy center were deemed eligible for sccSEMS placement. No patients who met eligibility criteria were denied sccSEMS placement. No patients were lost to follow-up. A total of 50 sccSEMS were placed. Piggy back placements were performed in 7 patients utilizing 16 sccSEMS. Piggy back placements were performed prior to the availability of dedicated small caliber esophageal SEMS in multiple lengths.
Descriptive data
The cohort consisted of 23 (74%) men and 8 (26%) women with a median age of 64 years (range, 35–87 years). Esophageal adenocarcinoma (AC) was present in 19 (61%) patients and SCCA in 12 (39%) patients. Of the patients with SCCA, 2 tumors were located in the proximal esophagus, 7 in the mid-esophagus and 3 in the distal esophagus. All 19 ACs were located in the distal esophagus. The AJCC clinical stages (21) at time of sccSEMS placement were: 1 (3%) Stage II, 8 (26%) Stage III, and 22 (71%) Stage IV. The initial pre-stent lumen diameter was less than 8 mm in 77% (24/31) of patients. The initial pre-stent lumen diameter was equal to 9 mm in 1 patient (3%), equal to 10 mm in 4 patients (13%), and equal to 11 mm in 2 patients (6%). The median pre-stent tumor length was 5.0 cm (interquartile range, 4.0–7.0 cm).
Outcome data
Dysphagia response
The DS improved in 97% (30/31) of patients who underwent sccSEMS placement (95% CI: 83–100%). A statistically significant decrease in the median pre- and post-stent DS was observed. The median pre-stent DS decreased from 3 (range, 3–4) to 2 (range, 1–3) after stent placement (P<0.0001). Placement of sccSEMS resulted in improvement of the pre-stent DS by a factor of two in 43% (13/31) of patients (95% CI: 25–61%). Food impaction did not occur. Recurrent dysphagia as a result of tumor overgrowth occurred in 13% (4/31) of patients at a median of 178 days (range, 95–249 days).
Migration rates and effective duration times
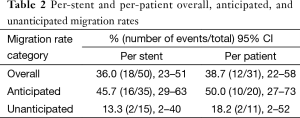
Migration rates were calculated on a per-stent and per-patient basis. Migration events were classified as either anticipated or unanticipated as previously defined. The unanticipated migration rates reflect sccSEMS migration rates in strictly palliative situations. A total of 15 stents were placed in 11 patients who subsequently received no tumor therapy. Two stent migration events occurred yielding a per-stent unanticipated migration rate of 13% (95% CI: 2–40%) and a per-patient unanticipated migration rate of 18% (95% CI: 2–52%) (Table 2). On a per stent basis, sccSEMS migration was 5.5 times (95% CI: 1.1–28.0) more likely to occur during tumor therapy than in the absence of tumor therapy.
Full table
We compared the overall migration rates of the various types of sccSEMS placed (Table 1). The biliary stent migration rate was 0% (0/2); tracheobronchial stent migration rate was 64% (9/14); and esophageal stent migration rate was 24% (8/34). The migration rate of tracheobronchial stents was significantly greater than the esophageal stent migration rate (P=0.018). Tracheobronchial stents were 5.9 times (95% CI: 1.5–22.6) more likely to migrate than esophageal stents.
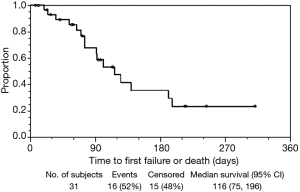
The median effective duration for the initial sccSEMS placement (EDS1) was 116 (95% CI: 75–196) days (Figure 2). At 30, 60, 90, and 120 days from initial sccSEMS placement, the estimated proportion of patients alive with a functioning initial stent was 93% (95% CI: 75–98%), 85% (95% CI: 66–94%), 68% (95% CI: 45–83%), and 48% (95% CI: 26–67%), respectively (Figure 2). For patients requiring endoscopic stent replacement due to migration or tumor stent overgrowth, the median total effective durations of all sccSEMS placed per patient (EDSt) was 196 (95% CI: 91–∞) days (Figure 3). At 30, 60, 90, and 120 days from initial sccSEMS placement, the estimated proportion of patients alive with a functioning stent was 97% (95% CI: 79–100%), 93% (95% CI: 75–98%), 74% (95% CI: 53–87%), and 62% (95% CI: 40–77%), respectively (Figure 3).
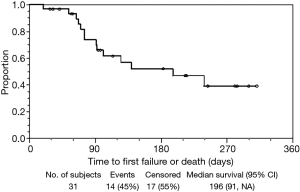
AE and death rates
We observed no instances of perforation, bleeding, fever, or fistulization related to sccSEMS placement. No deaths attributable to sccSEMS occurred. The major AE rate was 6.5% (Table 3). Severe chest pain post sccSEMS placement necessitated stent removal in one patient. One case of asymptomatic stent-induced esophageal pressure necrosis without esophageal perforation was observed. The minor AE rate was 19.4% (Table 3).

Full table
Technical success of sccSEMS placement and removal
Direct endoscopic placement of sccSEMS was successful in all cases. A total of 50 sccSEMS were placed during 41 separate procedures. The mean total procedure time was 23.9 (SD =13.6) minutes. The median total procedure time was 20 minutes (range, 7–62 minutes). Esophageal endoscopic ultrasonography was performed in conjunction with direct sccSEMS placement during 7 procedures. Excluding these procedures, the mean, median, and procedure time range required to perform sccSEMS placement was 19.8 (SD =10.5) minutes, 18 minutes, and 7 to 54 minutes, respectively. Ninety-four percent (17/18) of migrated sccSEMS were removed without complication. One stent migrated beyond the pylorus and passed spontaneously without complication.
Clinical outcomes
Patients were followed clinically from the time of last sccSEMS placement to the end of the study period. The median time to clinical follow-up from last sccSEMS placement was 125 days (interquartile range, 75–199 days). The final clinical outcomes are summarized in Table 4. A positive effective outcome from sccSEMS placement occurred in 93.5% (29/31) of patients. SccSEMS placement palliated dysphagia until death in 12 patients and continues to palliate dysphagia in 4 additional hospice patients. In 11 patients receiving local or systemic tumor therapy, sccSEMS controlled dysphagia in 7 patients until anticipated stent migration. The increase in esophageal lumen diameter observed at stent retrieval allowed for adequate swallowing without stent replacement in all 7 cases. SccSEMS placement controlled dysphagia until esophagectomy in one patient. The stent was removed with the surgical specimen.
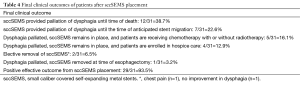
Full table
Discussion
In our study, malignant dysphagia was successfully managed with sccSEMS in 97% of patients with obstructing primary esophageal cancer. The observed sccSEMS unanticipated per-stent and per-patient migration rates were comparable to those reported for large caliber fully covered SEMS (lccSEMS) placed for palliation purposes (22,23). The median effective duration for first sccSEMS placement was 116 days. During follow-up, two major AEs were encountered. No stent related deaths occurred. In the management of malignant dysphagia, the safety of sccSEMS appears to substantially exceed that reported for lccSEMS (13,24-27). Under direct endoscopic visualization, sccSEMS were easily and rapidly placed and removed either from their original position within strictures or from the stomach across strictures after migration.
The role of esophageal stents in the management of malignant esophageal obstruction is changing. The paradigm of malignant dysphagia palliation as a unidisciplinary effort involving a single stent placement procedure at the end of life is waning (28). Stent placement alone may result in decreased quality of life compared to tumor reductive and ablative interventions (29). Improved chemotherapies, brachytherapy, and endoscopic tumor ablative interventions all have an increasing role in palliation. The development of removable covered SEMS permits stent placement and removal at any point in a sequence of multimodality therapies (30). Palliation is evolving into a multidisciplinary effort. A new “multimodality multidisciplinary management” paradigm is being established. Within this new paradigm, the safety and ease of esophageal stent deployment and removal will assume greater importance than the absolute degree of dysphagia relief achieved. Migration events will be analyzed with respect to cause rather than automatically attributed to stent malfunction.
Verschuur et al. (31) compared migration rates between 22–25 mm BD and 17–20 mm BD partially covered and covered SEMS placed for palliation of malignant dysphagia. Migration occurred in 3% of patients who received 22–25 mm BD stents, whereas, 15% who received 17–20 mm BD stents experienced migration. Among patients receiving 22–25 mm BD stents complications occurred in 23% and stent related deaths in 6%. For 17–20 mm BD stents complications occurred in 22% of patients and stent related deaths in 2%.
Comparison of historical data with our data provides evidence for increased safety of sccSEMS compared to lccSEMS in malignant dysphagia. Uitdehaag et al. (32) reported a 45 patient study of 18 and 22 mm BD lccSEMS (Alimaxx E) placed for treatment of malignant dysphagia. Major AEs (severe pain, hemorrhage, fever, and fistula) occurred in 20% of patients, minor AEs in 22%, and stent related deaths in 7%. Prospective studies of lccSEMS of different design from other manufactures have revealed similar AE rates (13,22,33). In the current 31 patient sccSEMS study, major AEs occurred in 6.5% of patients, minor AEs in 19.4%, and stent related deaths in no patients.
Although statistically significant, the magnitude of median dysphagia reduction in our study was less following placement of sccSEMS than that reported in studies of lccSEMS. Median DSs typically decreased by two levels when lccSEMS were placed (22,23,33-35). Median DSs in our sccSEMS patients decreased by only one level. Nonetheless, our sccSEMS patients experienced no food impaction events. This observation suggests that the frequency of food impaction events is primarily a function of dietary education and not stent BD. Our data appear to confirm that sacrificing a clinically inconsequential amount of dysphagia relief to BD reduction results in improved safety.
Direct endoscopic insertion of sccSEMS without fluoroscopy was rapid and safe. Previous reports of direct endoscopic stent insertion confirm our experience. Austin et al. (36) and Wilkes et al. (37) reported successful direct endoscopic placement of large caliber uncovered SEMS in 77% and 92% of cases respectively. Unlike Wilkes et al., we always attempted to traverse the stricture following stent deployment. In those instances where the combination of small caliber and stent wasting from a tight stricture prevented passage of the adult videogastroscope, the instrument was advanced to the point of gentle impaction and then withdrawn. These maneuvers were performed under the assumption that if the stent was not firmly seated in the stricture then it was better to immediately force migration, retrieve and redeploy then to bring the patient back later for a separate procedure to retrieve a spontaneously migrated stent.
We found endoscopic removal of migrated sccSEMS to be simple and rapid. All 17 sccSEMS that remained in the stomach after migration were easily removed without special equipment. In all instances, a standard adult single channel videogastroscope and a large or medium jaw rat tooth forceps were used. Early in our experience, we observed that the pull string took too much time to locate and grasp. With the edge grab and rotate maneuvers, it took less than 60 seconds to retrieve most migrated sccSEMS. We attribute the ease of sccSEMS retrieval to their small BDs and to the flexibility of the Alimaxx ES mesh design.
Our study has several limitations. The study was a single center small cohort study, and hence, the general applicability of our observations is unclear. Our study did not incorporate concurrent controls. To address the aims and objectives of our study, we compared our observational results to prior studies utilizing SEMS whose designs differed in aspects other than BD, and hence, the observed differences in safety, dysphagia relief, and migration rates between our study and prior studies may be due to design factors other than BD. Furthermore, the use of historical controls risks biases relating to patient selection, procedure technique, and accuracy of outcomes reporting. The initial use of sccSEMS designed for biliary and tracheal stenting may have introduced unfavorable performance data with respect to the subsequent use of sccSEMS specifically designed for esophageal use.
In conclusion, reduction in SEMS BD below 18 mm substantially decreases stent related AE and MRs compared to previously reported rates for SEMS with BDs over 18 mm. SccSEMS dysphagia reduction was adequate. Food impaction events were not observed. All sccSEMS were inserted safely and efficiently under direct endoscopic visualization. We observed a 5.5-fold increase in the odds of sccSEMS migration events during tumor therapy. We therefore categorized migration events as either anticipated or unanticipated depending on whether they occurred in the presence or absence of tumor therapy. We found elective removal of migrated sccSEMS to be safe and technically simple. Additional clinical studies are necessary to confirm our findings. As single modality unidisciplinary palliation of malignant dysphagia is abandoned in favor of multimodality multidisciplinary management, the ease and safety with which stents can be placed and removed from both their original position and from the stomach following migration will become of greater importance than the absolute degree of dysphagia relief achieved by stent placement. Research concerning effective integration of sccSEMS placement and removal into the multimodality multidisciplinary management of malignant dysphagia is needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tytgat GN, den Hartog Jager FC, Bartelsman JF. Endoscopic prosthesis for advanced esophageal cancer. Endoscopy 1986;18 Suppl 3:32-9. [Crossref] [PubMed]
- Earlam R, Cunha-Melo JR. Malignant oesophageal strictures: a review of techniques for palliative intubation. Br J Surg 1982;69:61-8. [Crossref] [PubMed]
- Fugger R, Niederle B, Jantsch H, et al. Endoscopic tube implantation for the palliation of malignant esophageal stenosis. Endoscopy 1990;22:101-4. [Crossref] [PubMed]
- Knyrim K, Wagner HJ, Bethge N, et al. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med 1993;329:1302-7. [Crossref] [PubMed]
- Siersema PD, Hop WC, Dees J, et al. Coated self-expanding metal stents versus latex prostheses for esophagogastric cancer with special reference to prior radiation and chemotherapy: a controlled, prospective study. Gastrointest Endosc 1998;47:113-20. [Crossref] [PubMed]
- Mosca F, Consoli A, Stracqualursi A, et al. Comparative retrospective study on the use of plastic prostheses and self-expanding metal stents in the palliative treatment of malignant strictures of the esophagus and cardia. Dis Esophagus 2003;16:119-25. [Crossref] [PubMed]
- De Palma GD, di Matteo E, Romano G, et al. Plastic prosthesis versus expandable metal stents for palliation of inoperable esophageal thoracic carcinoma: a controlled prospective study. Gastrointest Endosc 1996;43:478-82. [Crossref] [PubMed]
- Ramirez FC, Dennert B, Zierer ST, et al. Esophageal self-expandable metallic stents--indications, practice, techniques, and complications: results of a national survey. Gastrointest Endosc 1997;45:360-4. [Crossref] [PubMed]
- Adam A, Ellul J, Watkinson AF, et al. Palliation of inoperable esophageal carcinoma: a prospective randomized trial of laser therapy and stent placement. Radiology 1997;202:344-8. [Crossref] [PubMed]
- Vakil N, Morris AI, Marcon N, et al. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. Am J Gastroenterol 2001;96:1791-6. [Crossref] [PubMed]
- Bethge N, Sommer A, Gross U, et al. Human tissue responses to metal stents implanted in vivo for the palliation of malignant stenoses. Gastrointest Endosc 1996;43:596-602. [Crossref] [PubMed]
- Saranovic Dj, Djuric-Stefanovic A, Ivanovic A, et al. Fluoroscopically guided insertion of self-expandable metal esophageal stents for palliative treatment of patients with malignant stenosis of esophagus and cardia: comparison of uncovered and covered stent types. Dis Esophagus 2005;18:230-8. [Crossref] [PubMed]
- Kozarek RA, Raltz S, Brugge WR, et al. Prospective multicenter trial of esophageal Z-stent placement for malignant dysphagia and tracheoesophageal fistula. Gastrointest Endosc 1996;44:562-7. [Crossref] [PubMed]
- Ell C, Hochberger J, May A, et al. Coated and uncoated self-expanding metal stents for malignant stenosis in the upper GI tract: preliminary clinical experiences with Wallstents. Am J Gastroenterol 1994;89:1496-500. [PubMed]
- Siersema PD, Hop WC, van Blankenstein M, et al. A comparison of 3 types of covered metal stents for the palliation of patients with dysphagia caused by esophagogastric carcinoma: a prospective, randomized study. Gastrointest Endosc 2001;54:145-53. [Crossref] [PubMed]
- Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364:1497-504. [Crossref] [PubMed]
- Schaer J, Katon RM, Ivancev K, et al. Treatment of malignant esophageal obstruction with silicone-coated metallic self-expanding stents. Gastrointest Endosc 1992;38:7-11. [Crossref] [PubMed]
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007;147:W163-94. [Crossref] [PubMed]
- Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med 1985;145:1443-6. [Crossref] [PubMed]
- De Palma GD, Iovino P, Catanzano C. Distally migrated esophageal self-expanding metal stents: wait and see or remove? Gastrointest Endosc 2001;53:96-8. [Crossref] [PubMed]
- Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. Sixth edition. New York: Springer-Verlag, 2002.
- Uitdehaag MJ, Siersema PD, Spaander MC, et al. A new fully covered stent with antimigration properties for the palliation of malignant dysphagia: a prospective cohort study. Gastrointest Endosc 2010;71:600-5. [Crossref] [PubMed]
- Eloubeidi MA, Lopes TL. Novel removable internally fully covered self-expanding metal esophageal stent: feasibility, technique of removal, and tissue response in humans. Am J Gastroenterol 2009;104:1374-81. [Crossref] [PubMed]
- Sarper A, Oz N, Cihangir C, et al. The efficacy of self-expanding metal stents for palliation of malignant esophageal strictures and fistulas. Eur J Cardiothorac Surg 2003;23:794-8. [Crossref] [PubMed]
- Bartelsman JF, Bruno MJ, Jensema AJ, et al. Palliation of patients with esophagogastric neoplasms by insertion of a covered expandable modified Gianturco-Z endoprosthesis: experiences in 153 patients. Gastrointest Endosc 2000;51:134-8. [Crossref] [PubMed]
- Tomaselli F, Maier A, Sankin O, et al. Ultraflex stent--benefits and risks in ultimate palliation of advanced, malignant stenosis in the esophagus. Hepatogastroenterology 2004;51:1021-6. [PubMed]
- Homann N, Noftz MR, Klingenberg-Noftz RD, et al. Delayed complications after placement of self-expanding stents in malignant esophageal obstruction: treatment strategies and survival rate. Dig Dis Sci 2008;53:334-40. [Crossref] [PubMed]
- Ross WA, Alkassab F, Lynch PM, et al. Evolving role of self-expanding metal stents in the treatment of malignant dysphagia and fistulas. Gastrointest Endosc 2007;65:70-6. [Crossref] [PubMed]
- Shenfine J, McNamee P, Steen N, et al. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. Am J Gastroenterol 2009;104:1674-85. [Crossref] [PubMed]
- Barthel JS. Moving beyond single-modality management of malignant dysphagia. J Support Oncol 2008;6:274-5. [PubMed]
- Verschuur EM, Steyerberg EW, Kuipers EJ, et al. Effect of stent size on complications and recurrent dysphagia in patients with esophageal or gastric cardia cancer. Gastrointest Endosc 2007;65:592-601. [Crossref] [PubMed]
- Uitdehaag MJ, van Hooft JE, Verschuur EM, et al. A fully-covered stent (Alimaxx-E) for the palliation of malignant dysphagia: a prospective follow-up study. Gastrointest Endosc 2009;70:1082-9. [Crossref] [PubMed]
- Verschuur EM, Repici A, Kuipers EJ, et al. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: a randomized trial. Am J Gastroenterol 2008;103:304-12. [Crossref] [PubMed]
- Sabharwal T, Hamady MS, Chui S, et al. A randomised prospective comparison of the Flamingo Wallstent and Ultraflex stent for palliation of dysphagia associated with lower third oesophageal carcinoma. Gut 2003;52:922-6. [Crossref] [PubMed]
- Raijman I, Siddique I, Ajani J, et al. Palliation of malignant dysphagia and fistulae with coated expandable metal stents: experience with 101 patients. Gastrointest Endosc 1998;48:172-9. [Crossref] [PubMed]
- Austin AS, Khan Z, Cole AT, et al. Placement of esophageal self-expanding metallic stents without fluoroscopy. Gastrointest Endosc 2001;54:357-9. [Crossref] [PubMed]
- Wilkes EA, Jackson LM, Cole AT, et al. Insertion of expandable metallic stents in esophageal cancer without fluoroscopy is safe and effective: a 5-year experience. Gastrointest Endosc 2007;65:923-9. [Crossref] [PubMed]


