A rare case of three different tumors in the same pancreatic specimen: a case report and brief review of the literature
Introduction
The simultaneous presence of pancreatic exocrine and endocrine tumors, although rare, has been already reported (1-14). We report a case of simultaneous presence in the pancreas of a solid pseudopapillary tumor (SPT), a serous cystadenoma (SCA) and a neuroendocrine tumor (PanNET).
Case presentation
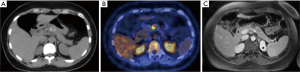
A 33-year-old Caucasian female was referred to our department for a solid nodule in the body of the pancreas in August 2014. Past medical records revealed several episodes of biliary colic due to gallstones treated surgically with laparoscopic cholecystectomy 1 year before. After a 6 months period of wellness, the patient started suffering from dyspepsia and epigastric nocturnal pain. She had no history of diabetes, smoking or alcohol abuse and did not use any drug on regular basis. There was no family history of pancreatic neoplasms. Biochemical tests did not reveal abnormalities. CA 19-9 was normal (<37 U/mL). An Ultrasonography confirmed the presence of a solid nodule in the body of the pancreas, which was further investigated with contrast-enhanced CT-scan (CT) (Figure 1A), magnetic resonance (MR) and endo ultrasonography (EUS). All the instrumental findings supported the diagnosis of SPT of the body of the pancreas. After admission, the patient underwent 18FDG PET/CT scan that revealed a pathologic uptake (Figure 1B) within the body of the pancreas (late maximum SUV was 5.6) with a peripheral calcified rim. To investigate whether the abnormal uptake was due to a neuroendocrine pancreatic tumor a 68Gallium DOTATOC PET/CT scan was performed. No areas of pathologic uptake were identified. Finally, a magnetic resonance cholangiopancreatography (MRCP) (Figure 1C) showed a hypovascular focal lesion located in the body of the pancreas, hyperintense on T1-weighted and hypointense in T2-weighted sequences. This lesion appeared inhomogeneous due to the presence of a peripheral calcified rim. The main pancreatic duct was lightly compressed and displaced by the lesion with a mild dilation upstream. Due to the symptoms and the suspected diagnosis of SPT the patient then underwent laparoscopic distal splenopancreatectomy. Post-operative course was complicated by grade B pancreatic fistula [according to ISGPF (15)], associated with fever, abdominal pain and leukocytosis, treated with parenteral nutrition and broad-spectrum antibiotics. The surgical pancreatic drainage was maintained and a second drainage was inserted for the presence of a fluid collection in the splenic lodge in 8th postoperative day. The patient was discharged in 32nd postoperative day.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Histopathologic analysis
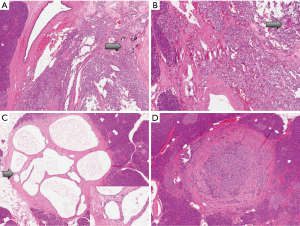
Pathological examination revealed a 2-cm SPT of the body of the pancreas (Figure 2A,B) without nodal involvement. Grossly, it was a gray-brownish lesion with a firm microcystic appearance. In the remaining pancreatic parenchyma two other neoplasms were discovered, a 7-mm serous cystadenoma (Figure 2C) and a 5-mm neuroendocrine microadenoma that grossly appeared as a brown firm nodule (Figure 2D).
Immunohistochemistry
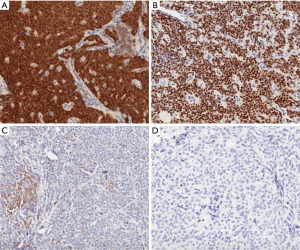
Immunohistochemical analysis was performed as previously described (16,17). Briefly, using 4 μm formalin-fixed paraffin-embedded sections, immunohistochemical analysis was conducted with the standard polymer system and peroxidase methods. After heat-induced antigen retrieval with a heated plate and 0.01 mol/L of citrate buffer, pH 8.9, for 15 min, all samples were processed using a sensitive ‘Bond Polymer Refine’ detection system in an automated Bond immunohistochemistry instrument (Vision-Biosystem, Leica, Milan, Italy). Sections incubated without the primary antibody served as negative controls. Immunostaining confirmed the diagnosis of solid pseudopapillary neoplasm for the main tumor as it showed nuclear positivity for β-catenin (Sigma, clone:15B8, 1:150 dilution) and progesteron-receptor (Dako, clone:PgR636, 1:20 dilution), cytoplasmic positivity for vimentin (Biogenex, clone:V9, 1:50 dilution) and CD10 (Novocastra, clone:56C6, 1:10 dilution), while it was negative for chromogranin A (Dako, clone:DAK-A3, 1:2,500 dilution) and had a scattered positivity for synaptophysin (Novocastra, clone:Snp88, 1:100 dilution). Ki-67 was <1% and 1 mitosis per 50 high power fields was detected (Figure 3). The neuroendocrine microadenoma was positive for chromogranin A and synaptophysin, as well as for CD56 (Thermo Scientific, clone:123C3.D5, 1:100 dilution), with a Ki67 proliferative index <1% (Novocastra, clone:MM1, 1:50 dilution).
Discussion
To our knowledge, this is the first report on the simultaneous presence of three different pancreatic neoplasms in a patient.
SPTs are rare pancreatic tumors accounting for 1–2% of all pancreatic exocrine tumors, are usually benign, but metastasis have been described in up to 15% of cases (18). Young women in the second decade are predominantly affected (19). There is no preferential localization within the pancreas, even though some case series report a more frequent localization in the body-tail of the pancreas (20). The origin of SPTs is still controversial. The most diffuse hypothesis is a differentiation from a pluripotential stem cell towards endocrine, acinar or epithelial-cell lineage (21,22). They have an indolent growth, which lead them to be discovered mostly incidentally or due to symptoms such as abdominal discomfort, palpable mass or weight loss. SPTs typically present radiologically as a large encapsulated mass with solid-cystic components and often intra-tumoral hemorrhage (23-25). While growing, SPTs can develop cystic changes, mimicking true pancreatic cystic tumors (26). At our Institution we routinely perform contrast enhanced CT-scan of the abdomen and MRCP when a SPT is suspected. With these radiological investigations a 95% preoperative diagnosis can be achieved (27). Surgical resection is the gold standard treatment for SPTs (20,28). Considering the excellent prognosis some reports suggest to resect also recurrences and metastases (26,29,30).
SCAs are rare non-mucinous cystic tumors with a benign behavior, with a stronger prevalence in females, and with a low growth rate (about 0.28 cm/year during the first 7 years from diagnosis); they are mostly incidentally found and often need differential diagnosis with mucinous cystic tumors. Follow-up of these lesions is the recommended strategy (31).
PanNETs measuring less than 0.5 cm are defined microadenomas and they are considered biologically benign. By definition these early stage PanNETs are nonfunctional (32). While they are common in patients suffering from MEN1 syndrome [so called “microadenomatosis” (33)], their prevalence as sporadic lesions ranges from 1% to 10% in adult population at autoptic findings (34). The diagnosis of endocrine microadenomas usually follows the pathological analysis of a pancreatic specimen resected for other reasons (32).
In conclusion, the association between pancreatic tumors originating from different cell lineages is already known. The most common reported combinations involve SCAs with PanNETs or ductal adenocarcinoma, and PanNETs with ductal adenocarcinoma (1-14). Yan et al. documented a 2-cm SPT of the tail of the pancreas engulfing a 0.7 cm well-differentiated PanNET (22). They postulated that a neoplastic pluripotential stem cell might differentiate in endocrine, epithelial and acinar cell lines.
This case report is unique, since three distinct neoplastic lesions, each derived from distinct cell lineages, have been found in the same pancreatic specimen. Considering the low prevalence of each of these pancreatic neoplasms, the probability to find them simultaneously is exceedingly rare. As a take home message, we want to underline the importance of a careful examination of the whole pancreatic specimen to rule out the possibility of other coexistent tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Blandamura S, Parenti A, Famengo B, et al. Three cases of pancreatic serous cystadenoma and endocrine tumour. J Clin Pathol 2007;60:278-82. [Crossref] [PubMed]
- Ustün MO, Tuğyan N, Tunakan M. Coexistence of an endocrine tumour in a serous cystadenoma (microcystic adenoma) of the pancreas, an unusual association. J Clin Pathol 2000;53:800-2. [Crossref] [PubMed]
- Alzaraa A, Udom V, Mousa H, et al. Combined endocrine and exocrine tumours of the pancreas. World J Surg Oncol 2007;5:103. [Crossref] [PubMed]
- Alasio TM, Vine A, Sanchez MA, et al. Pancreatic endocrine tumor coexistent with serous microcystic adenoma: report of a case and review of the literature. Ann Diagn Pathol 2005;9:234-8. [Crossref] [PubMed]
- Kim YW, Park YK, Lee S, et al. Pancreatic endocrine tumor admixed with a diffuse microcystic adenoma--a case report. J Korean Med Sci 1997;12:469-72. [Crossref] [PubMed]
- Heresbach D, Robert I, Le Berre N, et al. Cystic tumors and endocrine tumor of the pancreas. An unusual association. Gastroenterol Clin Biol 1993;17:968-71. [PubMed]
- Baek SY, Kang BC, Choi HY, et al. Pancreatic serous cystadenoma associated with islet cell tumour. Br J Radiol 2000;73:83-6. [Crossref] [PubMed]
- Goh BK, Tan YM, Kumarasinghe MP, et al. Synchronous serous cystadenoma and pancreatic endocrine tumor: a case report and literature review. Dig Dis Sci 2006;51:422-6. [Crossref] [PubMed]
- Agarwal N, Kumar S, Dass J, et al. Diffuse pancreatic serous cystadenoma associated with neuroendocrine carcinoma: a case report and review of literature. JOP 2009;10:55-8. [PubMed]
- Montag AG, Fossati N, Michelassi F. Pancreatic microcystic adenoma coexistent with pancreatic ductal carcinoma. A report of two cases. Am J Surg Pathol 1990;14:352-5. [Crossref] [PubMed]
- Slukvin II, Hafez GR, Niederhuber JE, et al. Combined serous microcystic adenoma and well-differentiated endocrine pancreatic neoplasm: a case report and review of the literature. Arch Pathol Lab Med 2003;127:1369-72. [PubMed]
- Hsieh MS, Liu KL, Tien YW, et al. Combined pancreatic endocrine tumor and serous cystadenoma. J Formos Med Assoc 2009;108:739-45. [Crossref] [PubMed]
- Keel SB, Zukerberg L, Graeme-Cook F, et al. A pancreatic endocrine tumor arising within a serous cystadenoma of the pancreas. Am J Surg Pathol 1996;20:471-5. [Crossref] [PubMed]
- Mohan H, Garg S, Punia RP, et al. Combined serous cystadenoma and pancreatic endocrine neoplasm. A case report with a brief review of the literature. JOP 2007;8:453-7. [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Luchini C, Parcesepe P, Nottegar A, et al. CD71 in Gestational Pathology: A Versatile Immunohistochemical Marker With New Possible Applications. Appl Immunohistochem Mol Morphol 2016;24:215-20. [Crossref] [PubMed]
- Luchini C, Parcesepe P, Mafficini A, et al. Specific expression patterns of epithelial to mesenchymal transition factors in gestational molar disease. Placenta 2015;36:1318-24. [Crossref] [PubMed]
- Klöppel G. Luttges J, Klimstra DS, et al. Solid-pseudopapillary neoplasm. In: Hamilton SR, Aaltonen LA, editors. WHO Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. Vol. 2. Lyon, France: IARC, 2000:241-8.
- Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 2005;200:965-72. [Crossref] [PubMed]
- Salvia R, Bassi C, Festa L, et al. Clinical and biological behavior of pancreatic solid pseudopapillary tumors: report on 31 consecutive patients. J Surg Oncol 2007;95:304-10. [Crossref] [PubMed]
- Canzonieri V, Berretta M, Buonadonna A, et al. Solid pseudopapillary tumour of the pancreas. Lancet Oncol 2003;4:255-6. [Crossref] [PubMed]
- Yan SX, Adair CF, Balani J, et al. Solid pseudopapillary neoplasm collides with a well-differentiated pancreatic endocrine neoplasm in an adult man: case report and review of histogenesis. Am J Clin Pathol 2015;143:283-7. [Crossref] [PubMed]
- Wang DB, Wang QB, Chai WM, et al. Imaging features of solid pseudopapillary tumor of the pancreas on multi-detector row computed tomography. World J Gastroenterol 2009;15:829-35. [Crossref] [PubMed]
- Choi JY, Kim MJ, Kim JH, et al. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol 2006;187:W178-86. [Crossref] [PubMed]
- Raman SP, Kawamoto S, Law JK, et al. Institutional experience with solid pseudopapillary neoplasms: focus on computed tomography, magnetic resonance imaging, conventional ultrasound, endoscopic ultrasound, and predictors of aggressive histology. J Comput Assist Tomogr 2013;37:824-33. [Crossref] [PubMed]
- Serrano PE, Serra S, Al-Ali H, et al. Risk factors associated with recurrence in patients with solid pseudopapillary tumors of the pancreas. JOP 2014;15:561-8. [PubMed]
- Salvia R, Malleo G, Marchegiani G, et al. Pancreatic resections for cystic neoplasms: from the surgeon's presumption to the pathologist's reality. Surgery 2012;152:S135-42. [Crossref] [PubMed]
- Yoon DY, Hines OJ, Bilchik AJ, et al. Solid and papillary epithelial neoplasms of the pancreas: aggressive resection for cure. Am Surg 2001;67:1195-9. [PubMed]
- Ng KH, Tan PH, Thng CH, et al. Solid pseudopapillary tumour of the pancreas. ANZ J Surg 2003;73:410-5. [Crossref] [PubMed]
- Tang LH, Aydin H, Brennan MF, et al. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol 2005;29:512-9. [Crossref] [PubMed]
- Malleo G, Bassi C, Rossini R, et al. Growth pattern of serous cystic neoplasms of the pancreas: observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut 2012;61:746-51. [Crossref] [PubMed]
- Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol 2007;20 Suppl 1:S94-112. [Crossref] [PubMed]
- Kloeppel G. Tumors of the endocrine pancreas. Vol. 2, Chapter 20. In: Fletcher CD, editor. Diagnostic Histopathology of Tumors, 4th Edition. 2013.
- Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci 1991;36:933-42. [Crossref] [PubMed]


