Restaging after neoadjuvant chemoradiation in rectal cancers: is histology the key in patient selection?
Introduction
Colorectal cancer is the third most common cancer worldwide, which contributes to 8% of cancer-related deaths worldwide (1). Survival rate for localized and locoregional cancers has improved over the decades that can be attributed to the development in effective adjuvant and neoadjuvant treatment strategies apart from early detection and screening protocols (2). Neoadjuvant chemoradiation (NACTRT) followed by radical resection with a total mesorectal excision (TME) approach has become the standard of care for locally advanced rectal cancers (LARC) as this has been shown to reduce overall mortality (3,4). The role of magnetic resonance imaging (MRI) of pelvis for local staging and contrast-enhanced computed tomography (CECT) of the abdomen and chest for metastatic workup have become mandatory for the initial staging of rectal cancer as per various international guidelines (5,6). Accurate staging leads to appropriate stratification into prognostic subsets with management tailored accordingly. However, not all patients who receive NACTRT progress to a successful radical resection, with a significant proportion of patients developing either local or systemic disease progression.
On the basis of the existing guidelines, clinicians are in favor of performing a restaging MRI pelvis after NACTRT to document response, predict circumferential resection margin (CRM) status, and plan surgery. However, no clear evidence is available to suggest whether restaging with CT scans is required to rule out systemic progression. Performing CT scans in all patients may not be cost-effective, unnecessarily exposes all the patients to radiation, and also possibility exists of complications due to intravenous contrast administration. We hypothesized that a subset of patients is at high risk of progressive disease, and restaging these patients alone will be more productive. We aimed to identify the factors that led to a higher incidence of disease progression.
Methods
This study was a retrospective audit of prospectively maintained data of all patients of LARC who were evaluated by the GI unit from August 2013 to April 2014 at Tata Memorial Centre, Mumbai, which is a tertiary cancer referral centre in India.
All patients were subjected to the routine staging workup comprising clinical evaluation, complete colonoscopy, MRI pelvis for local staging, and CT chest and abdomen (with oral and intravenous contrast) for systemic staging. All cases were discussed in a joint clinic comprising surgical, medical and radiation oncologists, radiologist, pathologist, nuclear medicine specialist, and medical gastroenterologist to plan treatment.
MRI protocol
Using a 1.5-T MRI scanner, we obtained T2W sequences without fat saturation in orthogonal planes to the tumor. Sagittal sequences were obtained first and then used to plan the coronal and oblique axial sections (perpendicular to the long axis of the rectum at the level of the tumor). CRM was considered positive if tumor/node/deposit/peritumoral stranding was located <1 mm from mesorectal fascia. CRM was considered threatened when this distance was between 1 and 2 mm. In low rectal tumors, CRM was measured from the tumor to the levator ani. Nodes were considered as involved if they were >5 mm or had irregular borders or mixed signal intensity on MRI (7).
Patients with (I) histologically proven adenocarcinoma; (II) node-positive/T4/advanced T3 (with CRM involved or threatened) on the basis of pretreatment MRI; and (III) no distant metastases on pretreatment staging CT chest and abdomen were included in this study. However, patients (I) with non-adenocarcinoma histology; (II) who have received any form of prior treatment (except for diversion stoma); (III) with recurrent tumors; and (IV) with distant metastases were excluded.
Chemoradiation protocol
External beam radiotherapy was delivered in fractions of 1.8–2 Gy to a total of 45–50 Gy over a period of 5–6 weeks using three fields or box fields. Concurrently, oral capecitabine was administered at a dose of 825 mg/m2 twice daily.
After radiation therapy, patients underwent a repeat MRI pelvis and were reevaluated at the joint clinic where decision was made regarding further treatment. All patients who were deemed to have a free CRM were planned for TME. Those who had CRM-positive tumors were planned for exenteration. However, in this latter group of patients, a whole-body positron emission tomography-computerized tomography (PET-CT) scan was performed to rule out distant metastasis before surgery. Patients who had gross unresectable disease were referred for palliative chemotherapy. Those who developed clinical signs of progression underwent confirmatory tests to document progressive disease. They were also referred for palliative chemotherapy.
Statistical analysis
The data were maintained prospectively, and the statistical analysis was carried out using SPSS software, version 20.0 (SPSS, Chicago, IL, USA). Categorical variables were analyzed using the Fisher’s exact tests.
Results
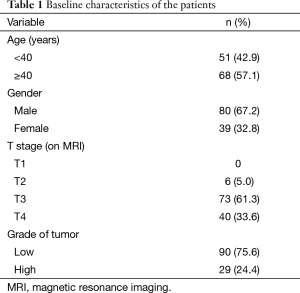
During the study period, 119 patients fulfilled the inclusion and exclusion criteria and were included in this audit. The baseline characteristics of the patients are outlined in Table 1.
Full table
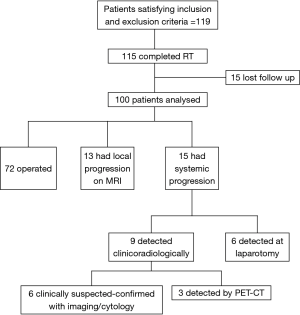
The median age was 44 years (range, 15 to 84 years). A significant proportion of the patients (61%) had T3 tumors. Grade was categorized into low and high (by grouping well and moderately differentiated tumors into low grade and poorly differentiated tumors into high grade) as suggested by the College of American Pathologists guidelines (8). Standard long-course chemoradiation was administered to all patients. Four patients could not complete radiation therapy because of complications. Fifteen patients were lost to follow-up after completion of radiation and could not be evaluated. Finally, 100 patients underwent reevaluation in the joint clinic.
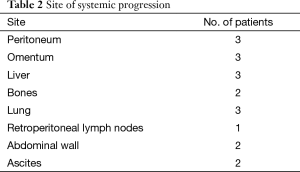
Figure 1 outlines the outcomes of the patients after NACTRT. Of 100 patients, notably, 15 patients progressed systemically whereas 13 had local progression. Table 2 shows the site of systemic progression. Few patients (4/15) had more than one site of metastatic disease.
Full table
Among the 15 patients who developed systemic progression, 6 had clinical symptoms of progression (2 patients had ascites, 2 developed abdominal wall nodules, and 2 developed bone pain). In these cases, disease was confirmed by ascitic fluid cytology, fine-needle aspiration cytology and CT scan, respectively. Three patients who underwent a PET-CT scan, as they had been planned for exenteration, were diagnosed with liver and lung metastasis. All patients who progressed were referred for palliative chemotherapy.
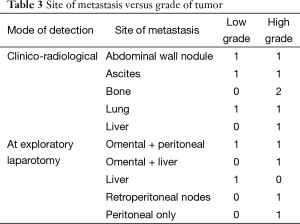
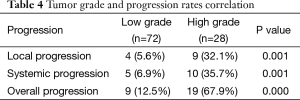
Table 3 shows the cross-tabulation of the site of metastasis versus grade of tumor. On correlating the grade of the tumor and progression of disease, the rates of local, systemic, and overall progression are found to be significantly more in the high-grade tumors than in the low-grade tumors (Table 4).
Full table
Full table
Discussion
NACTRT has become the cornerstone in management of LARC as it is known to reduce overall mortality and recurrence rates. In addition, it has been shown to cause tumor shrinkage, thus decreasing the local extent of disease and, in turn, the chances of positive CRM during surgery. However, there is a gap of 12–14 weeks between diagnosis and definitive surgery (considering the time taken for NACTRT and the subsequent waiting period) during which no meaningful chemotherapy is administered and, therefore, systemic progression can occur. Also, studies have shown that about 30–40% patients show no significant response to NACTRT, which is probably related to the tumor biology. This suggests that restaging before surgery should be considered.
There is conflicting data from various studies regarding the necessity of performing a restaging CECT scan of thorax and abdomen. Bisschop et al. (9) in a retrospective analysis of 153 patients of LARC from the Netherlands concluded that the final management plan was altered in 11.1% patients. A similar study by Ayez et al. (10) put this figure at 12% and also suggested that 8% of these patients could be spared radical surgery in view of progressive metastatic disease.
However, a recently published multi-institutional retrospective analysis concluded that only 6.7% patients had alteration of treatment plan (11). Another small study by Davids et al. (12) conducted on 83 patients of stage II and III rectal cancer reported that the management plan did not change in any of the patients, though 4 patients had new findings. Similarly, Jaffe et al. (13) reported that none of their 76 patients of nonmetastatic rectal cancer developed metastasis at the end of neoadjuvant therapy.
Our study shows that tumor grade predicts the rate of local, systemic, and overall progression, with more than two-thirds of the high-grade (poorly differentiated) tumors showing disease progression and hence not suitable to undergo TME surgery. These are the patients who are most likely to progress during NACTRT and thus benefit from restaging. Thus, when used selectively in this subset of patients, restaging can be made cost-effective.
Restaging could be performed using either PET scan or multidetector CT. PET scan although highly sensitive has low specificity. It is not useful in patients with mucinous histology (14) and also does not detect small volume disease, especially in the peritoneum. Multidetector CECT scan although a good modality for imaging the abdomen has its limitations as it is not very sensitive for detecting peritoneal disease. In our study, nine patients were diagnosed with metastatic disease because they either had clinical evidence of progression (abdominal nodule, ascites, bone pain) or were detected by PET scan performed before planning an extirpative surgery such as exenteration. Six patients were detected with metastases to omentum and peritoneum during surgery, which may have not been detected by the current imaging modalities. Thus, probably a combination of multidetector CECT scan with diagnostic laparoscopy may be the best possible tool for restaging, and this combination needs to be investigated in future studies. If this approach of performing a CECT scan with diagnostic laparoscopy had been applied only to the high-grade patients in our study, it would have been more productive and cost-effective as evidenced by the systemic progression rate of 35.7% in high-grade tumors as compared to 6.9% among low-grade tumors. This approach would have missed out only the two low-grade patients who had omental and liver metastases.
Multiple studies have proved that tumor grade is an important prognostic factor in rectal cancer. As per the consensus statement of the College of American Pathologists, tumor grade is a Category IIA prognostic factor influencing survival in colorectal carcinomas (CRCs) (8). They have recommended that all CRCs be grouped into low-grade (well/moderately differentiated) and high-grade (poorly/undifferentiated) tumors while making treatment decisions. We have observed in our study that approximately two-thirds (67.9%) of patients with high-grade tumors will have progression of disease and will not undergo definitive curative surgery. It is time to consider escalation of treatment strategies in this subset of patients. This might entail addition of chemotherapy either before or after NACTRT to prevent local and systemic progression of disease.
Limitations
Our study being retrospective in nature has its inherent limitations.
Occasionally, the initial biopsy sample may be insufficient to determine the grade of the tumor. It would be difficult to decide whether to restage these patients. Among these patients, it may be advisable to restage only those patients who had involved CRM initially and those who did not respond significantly to radiation (stable/progressive disease on MRI).
Conclusions
Restaging of patients with LARC after NACTRT could be restricted to high-grade tumors alone. This could prove more cost-effective and also avoid exposing the entire cohort to unnecessary radiation. These findings, however, need to be substantiated in a prospective trial using restaging workup in this high-risk group.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [Crossref] [PubMed]
- Wong RK, Tandan V, De Silva S, et al. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev 2007.CD002102. [PubMed]
- McCarthy K, Pearson K, Fulton R, et al. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev 2012;12:CD008368. [PubMed]
- Glimelius B, Tiret E, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi81-8. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Rectal cancer. Version 2. 2015. Available online: . Accessed 15 March 2015.http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
- Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003;227:371-7. [Crossref] [PubMed]
- Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979-94. [PubMed]
- Bisschop C, Tjalma JJ, Hospers GA, et al. Consequence of restaging after neoadjuvant treatment for locally advanced rectal cancer. Ann Surg Oncol 2015;22:552-6. [Crossref] [PubMed]
- Ayez N, Alberda WJ, Burger JW, et al. Is restaging with chest and abdominal CT scan after neoadjuvant chemoradiotherapy for locally advanced rectal cancer necessary? Ann Surg Oncol 2013;20:155-60. [Crossref] [PubMed]
- Hanly AM, Ryan EM, Rogers AC, et al. Multicenter Evaluation of Rectal cancer ReImaging pOst Neoadjuvant (MERRION) Therapy. Ann Surg 2014;259:723-7. [Crossref] [PubMed]
- Davids JS, Alavi K, Andres Cervera-Servin J, et al. Routine preoperative restaging CTs after neoadjuvant chemoradiation for locally advanced rectal cancer are low yield: a retrospective case study. Int J Surg Lond Engl 2014;12:1295-9. [Crossref] [PubMed]
- Jaffe TA, Neville AM, Bashir MR, et al. Is follow-up CT imaging of the chest and abdomen necessary after preoperative neoadjuvant therapy in rectal cancer patients without evidence of metastatic disease at diagnosis? Colorectal Dis 2013;15:e654-8. [Crossref] [PubMed]
- Berger KL, Nicholson SA, Dehdashti F, et al. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol 2000;174:1005-8. [Crossref] [PubMed]