Primary hepatic anaplastic large cell ki-1 lymphoma and celiac disease: a casual association?
Introduction
Although liver is one of the extranodal organs commonly involved in both Hodgkin and non-Hodgkin lymphoma, Primary Hepatic Lymphoma (PHL) is rare.
In non-immunocompromised patients, primary hepatic malignant non-hodgkin’s lymphoma is a rare disease, with less than 100 cases reported (1).
Anaplastic large-cell lymphomas (ALCL) were first described by Stein et al. in 1985 (2) as large-cell neoplasms with a pleomorphic appearance, subtotal effacement of the lymph node structure and expression of the lymphoid activation antigen CD-30 (ki-1). ALCL frequently involves both lymph nodes and extranodal sites (3). The most common extra-nodal sites affected by ALCL include skin, bone, soft tissue, lung, and liver. However, is extremely rare for ALCL to present as a liver primary lymphoma, and only eight cases have been reported.
ALCL accounts for approximately 3% of adult non-Hodgkin lymphomas. The neoplastic cells consistently express CD30 molecule in all variants. Most cases of ALCL are associated with the characteristic chromosomal translocation t[2;5], which results in up regulation of anaplastic lymphoma kinase (ALK) protein.
We report a case of a primary hepatic anaplastic large T-cell ki-1 non-Hodgkin lymphoma in a 55-year-old patient with celiac disease.
Case report
A 55 year old man followed during one year because of adult celiac disease was hospitalized due to fever, right upper quadrant abdominal pain and itch. The patient had no complaints of weight loss or night sweats.
Physical examination was normal. Laboratory results were normal, apart from an increase in liver function tests. Carcinoembryonic antigen (CEA) level and alpha feto protein (AFP) level were normal.
Epstein-Barr virus, hepatitis viruses and human immunodeficiency virus serologies were negative.
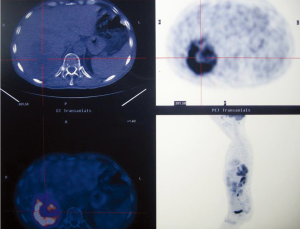
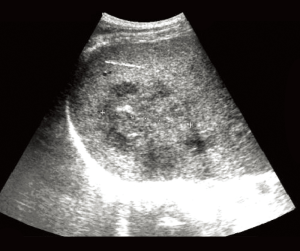
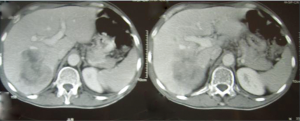
Abdominal ultrasound (Figure 1) and CT scan (Figure 2) showed an enlarged liver with a single mass in the right lobe (8.4 cm × 7.3 cm). No adenopathy was detected in the abdominal cavity, and there was a normal size and appearance spleen.
Percutaneous liver biopsy led the diagnosis of adenocarcinoma without differentiation.
Total body CT scan, PET-Scan (Figure 3), gastroscopy, colonoscopy and iliac crest bone marrow biopsy suggested a localized primary liver mass.
Because of the favourable anatomic location of the tumor and the absence of metastatic disease, a right hepatectomy was performed. The abdominal exploration revealed no evidence of extrahepatic tumor or adenopathy.
The postoperative course was favourable, and he was discharged from hospital on the 7th postoperative day.
The surgical specimen consisted of a right hepatic lobe of 1,500 g weight, with a neoplasm of 11 cm in segments 7 and 8. The tumor appearance was white, soft and homogeneous, without focal necrosis nor hemorrhage. It was well circumscribed and lobulated, and appeared to be completely within the limits of resection.
Microscopically, a population of lymphoid cells of large size with many mitotic figures was identified. The large neoplastic lymphoid cells immunostained positively for CD-30 (Ki-1), CD-3, ALC and CD-45, with an index of proliferation Ki 67 +++ (80%). The CD-20, CD-79a, CD-8, CD-4, CD-15, S-100, HMB45, AE1-3, CAM 5.2, actin/desmin tests were negative.
Therefore, the diagnosis of non-Hodgkin lymphoma, large-cell anaplastic type, Ki-1 lymphoma was given.
Postoperatively, the patient received systemic chemotherapy with cyclophosphamide, vincristine, doxorubicin and prednisolone (CHOP) for four courses.
Twenty months after surgery the patient is disease free.
Discussion
Non-Hodgkin lymphoma is a common lympho-proliferative disease. Liver involvement occurs in 10% of the patients and it means advanced disease (stage 4).
The first report of primary hepatic lymphoma was by Ata and Kamal in 1965 (4). Primary hepatic lymphomas (PHL) are rare, with fewer than 100 cases reported in the world literature. PHL defines an extra-nodal lymphoma of the liver without involvement of any other organ. PHL is rare, representing <1% of all extra nodal lymphomas (5). Most cases of PHL tend to be of B-cell origin with only a minority of them of T-cell origin (6-11). The occurrence of primary hepatic anaplastic large cell lymphoma is extremely rare. In our review of the literature, we identified only eight other cases of primary hepatic ALCL (12-16) (Table 1).
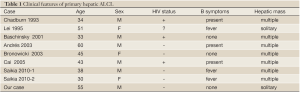
Full table
The ALCL are a clinically heterogeneous group of T, B and indeterminate cell malignant lymphomas. In the general population, ALCL has T-cell phenotype in 50-70% of cases, and the remainders are mostly null (non- T, non-B) phenotype. Rare cases of ALCL with B-cell phenotype are currently separated from T-cell or null-cell ACLC.
ALCL is a subgroup of diffuse large-cell lymphoma with characteristic morphology and strong expression of CD-30 (Ki-1) antigen. The Ki-1 antibody is a monoclonal antibody against the Hodgkin cell line L428, and at first it was regarded as an antibody specific for Hodgkin and Reed-Sternberg cells of Hodgkin disease. However, the Ki-1 antigen was also discovered later in patients with diffuse large cell-type non-Hodgkin lymphoma. The most frequently involved extranodal sites are skin, bone, soft tissue, GI tract and lung (6).
Most patients with PHL are middle-aged. Patients usually present with abdominal pain and constitutional symptoms. Hepatomegaly is found in the majority of patients (75-100%) and B symptoms (fever, drenching sweats and weight loss) appear in 37-86% of them (7). Less common presentations include ascites, hepatic failure, thrombocytopenia and hypercalcaemia.
PHL may present as a solitary liver mass (42%) or as multiple lesions (50%); diffuse infiltration of the liver is rare in Caucasians and more common in Chinese patients, but the pattern of the liver infiltration has no prognostic value (8).
The pathogenesis of PHL has not been established. An increasing number of cases are now being diagnosed in immuno-compromised patients, particularly patients with Human Immunodeficiency Virus. There is an association with hepatitis C (Hepatitis C infection is found in 60% of patients), which suggests that this virus may play a role in the pathogenesis of PHL, Epstein-Barr virus infection, immuno-suppression, organ transplantation, primary biliary cirrhosis and systemic lupus erythematosus.
Our patient neither virus infection nor signs of chronic liver disease were found.
Diagnosis of PHL requires the absence of lympho-proliferative disease outside the liver.
Liver biopsy of PHL may mimic poorly differentiated carcinoma, and in these cases, a high index of suspicion is needed. In our patient, liver biopsy did not confirm the diagnosis, and only the study of the surgical specimen gave the diagnosis of PHL.
Immuno-histochemical studies are required in order to distinguish between these tumours and poorly differentiated carcinomas. Sometimes further tests including cytogenetic studies, flow cytometry and gene rearrangement are necessary. However, detection of lymphomas by cytometry analysis is dependent of adequate sampling of the tissue, specimen viability and the ability of tumor cells to survive processing, and false negative results are common in large cell lymphomas (25% of large cell lymphomas are not detected by cytometry analysis). The main advantage of cytometry over immunohistochemical analysis in the initial diagnosis is speed, and may be helpful when the material for immunohistochemical analysis is not available, such as in the case of fine-needle aspiration (9).
ALCL is a T-cell lymphoma immunophenotypically characterized by positive CD30 staining in a membranous and Golgi zone pattern. Approximately 60-85% ALCL cases are positive for ALK protein. The translocation [2;5] between the ALK gene on chromosome 5 is the most common cytogenetic abnormality that leads to upregulation of ALK protein. The majority of ALC cases will show one or more T-cell-associated antigens. However, there are cases of ALCL that are negative for all T-cell markers and they are known as null-cell type.
The optimal treatment is not yet defined due to the low incidence of this disease. Surgery, radiotherapy and chemotherapy were all reported as treatment modalities alone or in combination (11). Only 17 patients, including our case, have been surgically treated. The importance of surgical resection for a cure or for a reduction of tumor burden cannot be assessed by this limited series. Whether or not systemic treatment with chemotherapy will give comparable results to surgery in resectable cases is also not currently known. Pescovitz et al. (10) noted that the disease-free survival rate for 5 patients treated with resection and combined chemotherapy was 80%, compared with 54% survival for chemotherapy alone. But Page et al. (7) reported complete remission in 83.3% of 24 cases evaluated with combination chemotherapy. Five year cause specific and failure free survival rates were 87.1% and 70.1% respectively. Outcomes are poorer in patients with AIDS, and in those with coexisting liver disease.
Currently, the most common treatment of PHL involves combination chemotherapy CHOP. Rituximab, an anti CD 20 monoclonal antibody, is added to this chemotherapy regimen if the histology confirms that the lymphoma is CD 20 antigen positive.
Massive liver infiltration, high index of proliferation, advanced age, elevated LDH levels, cirrhosis and increased levels of β2-microglobulin are the worst prognosis factors (12).
This is, to our knowledge, the first reported case of primary hepatic anaplastic large cell Ki-1 non-hodgkin lymphoma in a setting of adult celiac disease. The only link between both diseases is a markedly increased incidence of gastrointestinal T-cell lymphoma in celiac disease.
In conclusion, PHL is a rare disease, but it should be considered in any patient at any age who presents liver mass or infiltration, and, although hepatoma or metastatic diseases are more common, the absence of elevated levels of CEA and AFP should indicate a search for PHL.
Our patient had none of the classic predisposing factors for hepatic lymphocytic proliferation, such as chronic viral hepatitis, HIV, EBV or autoimmune hepatic disease.
However, other mechanisms for the development of NHL in celiac disease may be implicated. It is also possible that this may be an entirely fortuitous association.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Aozasa K., Mishima K, Ohsawa M. Primary malignant lymphoma of the liver. Leuk Lymphoma 1993;10:353-7.
- Stein H, Mason DY, Gerdes J, et al. The expression of Hodgkin’s disease associated antigen Ki-1in reactive and neoplastic lymphoid tissue. Evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cell. Blood 1985;66:848-58.
- Stein H, Foss HD, Durkop H, et al. CD30 (+) anaplastic large cell lymphoma: a review of its histopathologic, genetic and clinical features. Blood 2000;96:3681-95.
- Ata AA, Kamal IA. Primary reticulum cell sarcoma of the liver. A case report. J Egypt Med Assoc 1965;48:514-21.
- Lei KI. Primary non-Hodgkin’s lymphoma of the liver. Leuk Lymphoma 1998;29:293-9.
- Clavio M, Rossi E, Truini M, et al. Anaplastic large cell lymphoma: a clinicopathologic study of 53 patients. Leuk Lymphoma 1996;22:319-27.
- Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination chemotherapy. Cancer 2001;92:2023-9.
- OsborneBM, Butler JJ, Guarda LA. Primary lymphoma of the liver. Ten cases and review of the literature. Cancer 1985;56:2902-10.
- Kesler MV, Paranjape GS, Asplund SL, et al. Anaplastic large cell lymphoma: a flow cytometric analysis of 29 cases. Am J Clin Pathol 2007;128:314-22.
- Pescovitz MD, Snover DC, Orchard P, et al. Primary hepatic lymphoma in an adolescent treated with hepatic lobectomy and chemotherapy. Cancer 1990;65:2222-6.
- Scoazec JY, Degott C, Brousse N, et al. Non-Hodgkin's lymphoma presenting as a primary tumor of the liver: presentation, diagnosis and outcome in eight patients. Hepatology 1991;13:870-5.
- Avlonitis VS, Linos D. Primary hepatic lymphoma: a review. Eur J Surg 1999;165:725-9.
- Andrès E, Perrin AE, Maloisel F, et al. Primary hepaticanaplastic large cell Ki-1 non-Hodgkin’s lymphoma and hereditary hemochromatosis: a fortuitous association? Clin Lab Haematol 2003;25:185-6.
- Lei KI, Chow JH, Johnson PJ. Aggressive primary hepatic lymphoma in Chinese patients. Presentation, pathologic features, and outcome. Cancer 1995;76:1336-43.
- Bronowicki JP, Bineau C, Feugier P, et al. Primary lymphoma of the liver: clinical-pathological features and relationship with HCV infection in French patients. Hepatology 2003;37:781-7.
- Saikia UN, Sharma N, Duseja A, et al. Anaplastic large cell lymphoma presenting as acute liver failure: a report of two cases with review of literature. Ann Hepatol 2010;9:457-61.