Determining the optimal number of lymph nodes harvested during esophagectomy
Introduction
Esophageal cancer (EC) remains a highly lethal malignancy in the United States, with an estimated 5-year survival rate of 10–13% (1). Despite incorporating chemotherapy and/or radiotherapy into the treatment of locally advanced EC, esophagectomy remains an essential part of treatment paradigm especially when performed in a high volume institution (2). The role of adjuvant therapy is at best inconclusive.
Tumor depth and nodal status are critical for adequate staging of EC. Assessment of a higher number of lymph nodes (LNs) during curative resection has been shown to correlate with improved survival in patients with gastric cancer (3), cholangiocarcinoma (4) and pancreatic cancer (5). In EC, the extent of lymphadenectomy and the degree to which LN should be sought remain controversial. Evaluation of LN is not uniform, and it varies between laboratories and individual pathologists.
Supporters of extended lymphadenectomy quote impressive therapeutic benefits and long-term survival when three field lymphadenectomy is performed. However, retrospective series evaluating the association between LN resected during esophagectomy and survival have yielded conflicting results. Patients included in these series were heterogeneous, the majority underwent esophagectomy without neoadjuvant therapy and in some cases patients received adjuvant therapy. In Asian countries, where squamous cell carcinoma (SCC) is the most common pathology, three-field lymphadenectomy of the mediastinal, abdominal and cervical nodes is commonly used with low mortality and morbidity, improvement in local control and or survival is uncertain. Multiple series have been published in this setting with conflicting results (6-9).
The above series were based entirely on patients who underwent surgical resection without standardized peri-operative treatment which affect its validity given that neoadjuvant therapy is the current standard of treatment for locally advanced EC at least in North America. Other factors to be taken into accounts include: advances in staging modalities, stage migration and the temporal variation in incidence between the two major histological types of EC.
The objective of our study is to evaluate the clinical relevance of the number of LN resected during esophagectomy on recurrence and survival for patients with EC in the era of neoadjuvant therapy.
Methods
Patients
Following IRB approval, the gastrointestinal department at Moffitt Cancer Center (MCC) established a database of esophagectomy cases by performing a retrospective chart review of patients operated on at MCC between June 1994 and March 2011. The data collected included patient demographics, preoperative symptoms, Charlson comorbidity index (10), risk factor history, family history, tumor stage and histopathologic features, peri-operative events and complications. Chart reviews were performed solely by experienced clinicians and recorded on standardized abstraction forms.
All patients included in our database underwent staging with physical exam, EGD, endoscopic ultrasound (EUS), CT scans, CT/PET or PET scans. All pathology specimens from the initial endoscopic biopsies were read and confirmed by pathologist with specialization in gastrointestinal malignancies. Patients were seen and examined every 3 months for the first 2 years, then at every 6 months for years 2–5, and then annually. Routine follow-up exams included, physical exam, history, CT scans of chest/abdomen and pelvis. Endoscopy was performed if clinically indicated.
For this study, the database was queried according to our inclusion criteria for all patients who completed an esophagectomy. We analyzed our data using four categories based on the number of LN harvested at time of esophagectomy; <8, 9–12, 13–20, >20. The choice of nodal cut points were made based upon existing data for other malignancies, colorectal [12] and gastric [15] as well as the world EC coalition recommendations that >20 LNs should be removed for EC T2 or greater (3,4).
Statistical methods
Statistical analyses performed included univariate analysis of age, sex, pre-therapy tumor stage, histology and histological grade, type of esophagectomy, and number of LN resected. For these analyses we used chi-squared or Fisher’s exact tests, as appropriate for categorical data and mean differences were examined for continuous data using ANOVA. Survival analyses included analysis of all patients with adenocarcinoma (AC) and SCC, AC tumor only, and SCC tumors only by each LN category. For these analyses we used Kaplan-Meier Curves with Log Rank tests for significance. All statistical tests performed were two-sided and declared at the 5% significance level. Cox Proportional-Hazards Regression was performed to estimate the magnitude of association [odds ratio (OR)] and 95% confidence interval (CI) of each LN category. All multivariate analyses were controlled for: age, gender, pre-therapy tumor stage, histology, and neoadjuvant chemoradiation status. Statistical analyses were performed with STATA IC (STATA Statistical Software, Release 10.0; Strata Corp., College Station, TX, USA).
Surgical resection
All operations were performed with curative intent. Surgical approaches to esophagectomy included transthoracic, thoracoabdominal, and transabdominal techniques. Esophagogastrectomy was defined as resection of the proximal stomach and thoracic esophagus with an esophagogastric anastomosis. Minimally invasive esophagectomy (MIE) via transhiatal or transthoracic technique was performed on select patients. The choice of operation was based on the site of the primary tumor and surgeon preference.
In the right-sided transthoracic approach, esophagectomy and mediastinal LN dissection were performed in the thoracic stage. Esophageal substitute mobilization and celiac lymphadenectomy were performed in the abdominal stage. The thoracoabdominal approach included simultaneous exposure of the left-sided pleural cavity and abdominal cavity. The principle of dissection in the left-sided thoracoabdominal approach was similar to that of the right-sided transthoracic esophagectomy. The transhiatal approach included celiac lymphadenectomy and blunt dissection from the diaphragmatic hiatus to the thoracic inlet.
Pathological examination
LN groups were labeled according to the location and the American Joint Committee on Cancer (AJCC) LN classification system. All LN were cut at a thickness of 5 µm, embedded in paraffin, and sectioned for H&E staining. Descriptions of the tumor and the LN were recorded. The recorded number of LN is the sum of nodes collected at each level.
Results
Demographics, surgical resection, LNs, recurrence and survival
There were 709 patients in the esophageal database of which 635 met the criteria for inclusion in the study. The population consisted of 541 males and 94 females with a median age at diagnosis of 65 years (range, 28–86 years) and a median follow-up of 22 months [0–168]. AC [559] was the predominant histology (88%), whereas SCC [76] reflected only 12% of the cases (Table 1).

Full table
Four hundred and eighteen patients (66.7%) had node negative disease and 209 (33.3%) had nodal metastases at the time of surgery. There was a significant difference in the number of total nodes resected by trans-thoracic compared with the number of nodes resected by transhiatal (P=0.0320). There were 171 (27.5%) recurrences with a median time to recurrence of 12.2 months. Overall 5-year survival was 56.3% for node negative patients and 21.9% for those with nodal metastases (P<0.0001).
Impact of number of LNs resected
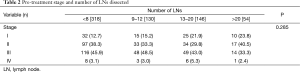
There was no association between the number of LN resected during esophagectomy and the pre-treatment clinical stage (Table 2). The 5-year overall survival (OS) and disease-free survival (DFS) rates for the group by LN category (<8, 9–12, 13–20, and >20 LN) were (43%, 42%, 55%, and 36%, P=0.1836) and (44%, 37%, 46%, and 36%, P=0.5166), respectively (Figure 1). Total number of LN assessed did not correlate with reduced risk of recurrence or survival.
Full table
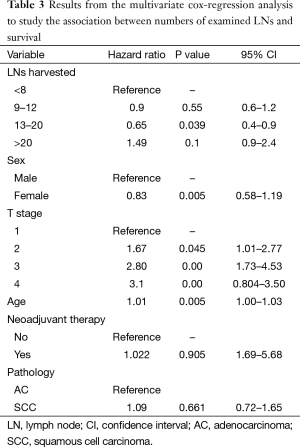
In the multivariate analysis, the total number of LN assessed again failed to demonstrate a correlation with decreased risk for recurrence and improved OS in 3 out of 4 categories. Patients who had 13–20 LN harvested demonstrated an improved survival compared to all other nodal cohorts (HR 0.65, P=0.039). Increasing age and higher T stage was associated with increased mortality, while gender, pathology (AC vs. SCC) and neoadjuvant therapy were not (Table 3).
Full table
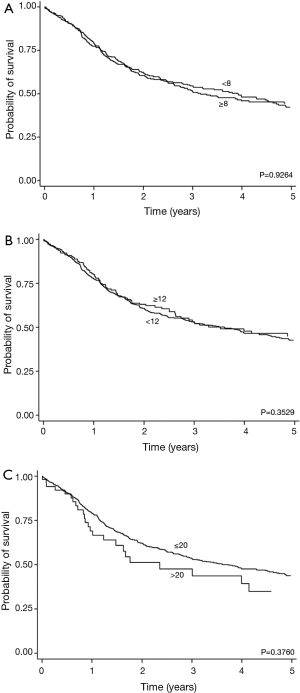
We further examined the significance of LN removed using absolute cut off points of ≤8 vs. >8, ≤12 vs. >12 and ≤20 vs. >20. There was no improvement in survival noted between those who had an increase number of LN harvested using cut off points of 8, 12, or 20 (Figure 2). Additionally we evaluated the association of number of LN sampled, PFS and OS based on pathology (AC vs. SCC), whether LNs were positive or negative after surgery, or whether patients received neoadjuvant therapy.
AC vs. SCC
The total number of patients with AC was 559. The number of patients in each group (<8, 9–12, 13–20, and >20 LN) was [266, 116, 128, 48]. The 5-year OS and DFS rates broken down by LN category were (43%, 43%, 56%, and 34%, P=0.1663) and (44%, 37%, 46%, and 33%, P=0.4881), respectively. In the SCC group we had 76 patients. The 5-year OS and DFS rates by LN category (<8, 9–12, 13–20, and >20 LN) were (46%, 35%, 49%, and 50%, P=0.4169) and (47%, 36%, 44%, and 75%, P=0.4674), respectively.
Nodal status at surgery
At the time of esophagectomy, 209 patients had LN positive disease. The 5-year OS and DFS rates by LN category (<8, 9–12, 13–20, and >20 LN) were (17%, 31%, 21%, and 27%, P=0.4372) and (17%, 23%, 16%, and 25%, P=0.2726), respectively. In the node negative group, the total number of patients was 418. The 5-year OS and DFS rates by LN category (<8, 9–12, 13–20, and >20 LN) were (54%, 51%, 79%, and 26%, P=0.0538) and (55%, 48%, 64%, and 27%, P=0.3703), respectively.
Neoadjuvant vs. no neoadjuvant therapy
We identified 246 (38.7%) patients who underwent esophagectomy as initial treatment without neoadjuvant therapy and 389 (61.3%) who were treated with neoadjuvant therapy. There was no correlation between number of LNs resected on overall or disease free survival in patients treated with esophagectomy without neoadjuvant therapy (Results not shown).
Discussion
We report our series of 635 patients at a single institution examining the impact of nodal sampling on outcomes in patients with EC. We demonstrated that the transhiatal approach to esophagectomy had statistically lower number of LNs removed (P=0.032) compared to the transthoracic approach. While there were no absolute cut points for nodal count that correlated to improve survival, patients that had 13–20 LN’s removed had improved survival (P=0.039) compared to all other groups. Additionally, no differences were noted in histologic sub type (EAC or SCC) or neoadjuvant therapy and nodal harvest. Moreover, no differences in overall or disease free survival were noted in the node positive and node negative groups with increasing LNs resected.
The current surgical guidelines have not yet established an optimal number of LN to be removed during esophagectomy. In a series of 5,620, patients with EC (11), the total LN was an independent prognostic variable; higher total LN (>30) and negative LN count (>15) category was associated with better OS and lower 90-day mortality. These effects were independent of nodal status or histology. These patients did not receive neoadjuvant therapy, and 48% did not receive radiation therapy. Data on chemotherapy was not available.
In another surveillance, epidemiology and end results (SEER) analysis of 2,303 EC patient (1,381 AC, 922 SCC) (12), the number of LN resected was found to be an independent predictor of survival after esophagectomy as well and the author suggested that to maximize survival benefits, a minimum of 23 LN needed to be removed. Unfortunately the SEER database lacks information on performance status, margin status, adjuvant chemotherapy, dosage of radiation, and radiation field design. Although the authors excluded patients receiving neoadjuvant and adjuvant therapies in their study, other potentially confounding factors cannot be controlled for and therefore caution should be used in the interpretation of these results.
In a smaller series of 268 patients with esophageal SCC who did not receive neoadjuvant therapy, the total number of resected LN was found to be predictor of OS in patients with N0 disease only (13). Recently, a series of 4,627 patients who had esophagectomy alone for EC (14) from a worldwide collaboration database was reported. The extent of lymphadenectomy was associated with increased survival for all patients except for early stage disease, or when seven regional LN were positive for cancer. Recommendations were made for nodal harvest based upon T-stage. However, this data is compiled from multiple institutions which make it difficult to control for pathologic examination, and surgical variation. Also, given that patients receiving neoadjuvant therapies were excluded, extrapolation of their recommendations for all EC patients should not be performed.
Supporters of extended lymphadenectomy quote impressive therapeutic benefits and long-term survival when three field lymphadenectomy is performed. In a series of 80 patients with AC and SCC of the esophagus (15); the overall 5-year survival rate was 51% and 30% of patients were upstaged to stage IV disease secondary to cervical LN involvement. Advanced staging modalities, stage migration, heterogeneity of patients included (tumor location in particular) and low incidence of cervical nodal involvement following two-field esophagectomy negatively impact the conclusion of the study (16,17).
In a series of 972 EC patients who underwent surgery for LN-negative disease from the SEER cancer registry (18), the 5-year disease specific survival was higher in patients who had more than 18 negative LN removed during esophagectomy. The study suggested that patients should have at least 18 LN removed during surgery, however, all patients had LN negative disease, did not receive neoadjuvant therapy, and 12% of them received post-operative radiation therapy.
Two randomized trials compared different extents of lymphadenectomy during esophagectomy; a prospective trial from the Netherlands which randomly assigned 220 patients with mid to lower esophageal AC to transhiatal resection or a transthoracic resection with an extended en bloc lymphadenectomy of mediastinal, upper abdominal nodes, and nodes in the aorto-pulmonary window (19). Patients did not receive neoadjuvant therapy. Perioperative morbidity was higher after transthoracic resection, and after a median follow-up of 4.7 years, there was a trend towards better disease-free and OS for patients undergoing transthoracic en-bloc esophagectomy, but neither was statistically significant. A second prospective trial comparing extended versus conventional lymphadenectomy in patients undergoing esophagectomy was hard to interpret as the study randomized only 73 of a potential 264 eligible patients, and following surgery, patients were randomly assigned to one of three groups for adjuvant therapy (20) .
In our study, patients with fewer than 8 total LN harvested did not exhibit higher rates of recurrence and death when compared with patients with 20 or more total LN. However, when examining this impact of lower nodal harvest on patients with node negative and node positive disease, there was clearly a decrease in survival in patients that were node positive who had fewer than 8 LNs resected compared to the other cohorts 17% vs. 31% [9–12], 21% [13–20], and 27% (>20). This was not seen in the node negative patients.
Our results corroborate findings from a previously published prospective randomized trial demonstrating no significant survival difference between two-field and three-field lymphadenectomies, however, additional prospective trials addressing the extent of lymphadenectomy are lacking. As mentioned above multiple retrospective population based studies (3-5,11-14,18) suggested that extended lymphadenectomy can improve outcome in EC patients, however, these studies were biased as they lacked uniform staging procedures, treatment approach and standard pathological evaluation. Additionally, the majority of patients analyzed in these series did not undergo any form of neoadjuvant or adjuvant therapy.
Although the sample size in this study is small compared to larger previously published series, our single-institution data is more uniform and complete and contains information that is not present in some of the larger databases. All of our patients underwent surgical resection and the majority received some form of neoadjuvant or adjuvant therapy which is considered the standard of care for patients with locally advanced disease.
The number of harvested LNs early in our experience was lower compared to other series. Considering the majority of patients 389 (61%) received chemoradiation, this could explain the lower LN harvest. Additionally, 121 (19.1%) of patients underwent a transhiatal resection which resulted in significantly lower LN harvest compared to those who underwent transthoracic approach. Interestingly, the average number of LNs included in the specimen did increase significantly following the implementation of routine fat-clearing techniques by our pathologist in 2007, instituting robotic and total minimally invasive techniques by our surgeons in 2010 and a shift from the transhiatal approach to trans-thoracic approach (8.87±5.8 in 1995–2006 vs. 14.1±7.8 in 2007–2010, and 20.4±9.4 in 2010-present P<0.0005).
Conclusions
In this large population of patients with EC at a tertiary care referral center, we have demonstrated that while no absolute number of LNs removed correlated with improved survival, those patients with 13–20 resected LNs did have improved overall and disease specific survival and removal of fewer than 8 LNs in patients with node positive disease resulted in decreased survival. While the importance of standardized pathologic examination and adequate nodal staging is of utmost importance for patients with EC undergoing esophagectomy the optimum number of LNs removed clearly warrants further investigation particularly in the setting of neoadjuvant therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117-27. [Crossref] [PubMed]
- Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005;23:7114-24. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Lymph node dissection impact on staging and survival of extrahepatic cholangiocarcinomas, based on U.S. population data. J Gastrointest Surg 2007;11:158-65. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol 2006;13:1189-200. [Crossref] [PubMed]
- Tachibana M, Kinugasa S, Yoshimura H, et al. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. Am J Surg 2005;189:98-109. [Crossref] [PubMed]
- Watanabe H, Kato H, Tachimori Y. Significance of extended systemic lymph node dissection for thoracic esophageal carcinoma in Japan. Recent Results Cancer Res 2000;155:123-33. [Crossref] [PubMed]
- Osugi H, Takemura M, Takada N, et al. Prognostic factors after oesophagectomy and extended lymphadenectomy for squamous oesophageal cancer. Br J Surg 2002;89:909-13. [Crossref] [PubMed]
- Shim YM, Kim HK, Kim K. Comparison of survival and recurrence pattern between two-field and three-field lymph node dissections for upper thoracic esophageal squamous cell carcinoma. J Thorac Oncol 2010;5:707-12. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable esophageal cancer. J Gastrointest Surg 2007;11:1384-93; discussion 1393-4. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Hsu PK, Wang BY, Chou TY, et al. The total number of resected lymph node is not a prognostic factor for recurrence in esophageal squamous cell carcinoma patients undergone transthoracic esophagectomy. J Surg Oncol 2011;103:416-20. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. [Crossref] [PubMed]
- Hulscher JB, Van Sandick JW, Offerhaus GJ, et al. Prospective analysis of the diagnostic yield of extended en bloc resection for adenocarcinoma of the oesophagus or gastric cardia. Br J Surg 2001;88:715-9. [Crossref] [PubMed]
- Dresner SM, Griffin SM. Pattern of recurrence following radical oesophagectomy with two-field lymphadenectomy. Br J Surg 2000;87:1426-33. [Crossref] [PubMed]
- Greenstein AJ, Litle VR, Swanson SJ, et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer 2008;112:1239-46. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 1998;175:47-51. [Crossref] [PubMed]


