Neoadjuvant chemoradiation followed by orthotopic liver transplantation in cholangiocarcinomas: the emory experience
Introduction
Cholangiocarcinoma (CCA) is a bile duct tumor with a grim prognosis (1,2). It accounts for 3% of all gastrointestinal malignancies (3). The incidence of CCA in the United States has increased and is currently estimated at approximately 3,500 cases per year (3,4). Patients with primary sclerosing cholangitis (PSC) are particularly prone to develop CCA (1,2). Anatomically, CCA is defined by disease occurring above the junction of the cystic duct up to the secondary branches of the right and left hepatic ducts. CCA occurs in two forms: (I) an intrahepatic mass separate from the hilus and major biliary branches, best treated by liver resection, or (II) presenting with obstruction of either the extrahepatic and/or major intrahepatic bile ducts (5,6).
Until recently, surgical resection including a partial hepatectomy has been the mainstay of treatment for periductal CCA arising in the liver hilus. This approach has led to a 5-year overall survival (OS) of 20-30%. Nonetheless, having extensive perineural and lymphatic invasion, bilateral liver involvement, and vascular encasement make some patients ineligible for this technique (7-10).
Orthotopic liver transplantation (OLT) achieves a tumor-free margin within the liver, accomplishes a radical resection, and also treats underlying PSC. However, high recurrence rates and poor OS were observed during the late 1980s and early 1990s. Addition of pancreatoduodenectomy at the time of OLT did not yield better results (11,12).
Despite the poor outcomes with OLT, patients with negative margins and absence of regional lymph node metastases did benefit (11). Furthermore, a small group of patients at the Mayo Clinic treated with primary radiotherapy and chemosensitization alone had a 22% 5-year OS (13). Based on this point as well as that CCA resection failures are usually due to locoregional recurrence, the University of Nebraska studied use of neoadjuvant (NEO) brachytherapy and 5-fluorouracil (5-FU) followed by OLT. Early results were promising with regard to locoregional control of cancer. Eleven patients transplanted for CCA following NEO therapy demonstrated 45% tumor-free survival (TFS) with a median follow-up of 7.5 years (14). The Mayo clinic adopted this concept with the development of a similar NEO therapy/liver transplant protocol in 1993. Preliminary results showed an 82% 5-year survival for 28 patients. Among the exclusion criteria in their series included: intrahepatic CCA, prior radiation or chemotherapy, prior biliary resection or attempted resection, evidence of extrahepatic disease, and history of other malignancy within 5 years (15).
Given these promising published results, we proceeded, in 2008, to treat the first CCA patient in our institution with NEO chemoradiation followed by OLT. The following is a review of our experience.
Methods
Following institutional review board approval, we retrospectively reviewed data obtained in ten consecutive patients with ten CCAs who were selected as candidates for NEO-OLT between 2008-2011. Diagnoses of CCA were established by intraluminal brush cytology, intraluminal biopsy, or a carcinoma antigen (CA) 19.9 level greater than 100 ng/mL in the setting of a radiographic malignant stricture.
All patients with de novo CCA were evaluated for resectability by an experienced hepatobiliary surgeon. Consensus of whether to perform NEO-OLT was achieved after discussion with the transplant surgeon, gastroenterologist, medical oncologist and radiation oncologist assigned to the case. Clinical staging prior to NEO included chest and abdomen computed tomography (CT), liver ultrasound, and bone scan. All patients underwent endoscopic ultrasound with fine needle aspiration of suspicious lymph nodes. Exclusion criteria included previous chemotherapy or radiotherapy, uncontrolled infection, a previous malignancy other than skin or cervical cancer within 5 years, medical conditions precluding transplantation, extrahepatic disease (including regional lymph node involvement), and operative biopsy or attempted resection of the tumor.
Vascular encasement and tumor size were not included in exclusion criteria. Patients with unresectable CCA above the cystic duct without intra or extrahepatic metastases were eligible. PSC patients were included due to their poor resection response. Patients initially received external-beam radiation [via conventional fields or volumetric-modulated arc therapy (VMAT)] to a target dose of 4,500 cGy in 30 fractions plus capecitabine (XEL) or 5-FU, followed by either Iridium192 (Ir192) brachytherapy high dose rate (HDR) or external boost. If HDR was utilized, a transluminal boost of radiation was delivered using a transcatheter Ir192 brachytherapy wire with a target dose of 2,000-3,000 cGy. External boost was delivered via either VMAT or conventional fields. 5-FU was given at a continuous infusion dose of 225 mg/m2. XEL was administered in the following manner: 2,000 mg/m2 per day in two divided doses, 2 out of every 3 weeks. 5-FU or XEL was administered until OLT. Patients underwent periodic surveillance abdominal CT or magnetic resonance imaging after OLT. Primary endpoints included actuarial rates (AR)/crude rates (CR) of OS, and local control (LC) at 6, 12, and 24 months.
All patients underwent a staging operation before transplantation. Extrahepatic metastases, lymph node metastases, and local extension of disease to adjacent organs or tissues precluded liver transplantation.
Only patients with operatively confirmed stage I or II CCA underwent OLT. OLT was performed with deceased donor livers, living donor right livers, and a familial amyloid domino donor liver.
Statistical analysis
Comparisons between continuous variables were performed using the Students’ t-test. OS and LC AR were calculated using the Kaplan-Meier method. P values less than 0.05 were considered statistically significant. The Biostatistics Department of Emory University’s Rollins School of Public Health (JV) conducted all statistical analysis.
Results
Demographics/follow-up assessment
Five males and five females were identified who were eligible candidates for treatment with NEO-OLT. The mean age was 58.3 years (range, 38-71 years) and median composite radiation dose (including boost) was 59 Gy (range, 54-71.4 Gy). Four patients underwent an HDR boost (40%), while six patients (60%) underwent an external boost. Of the external boost patients, four of them underwent conventional radiotherapy, while two others underwent VMAT.
Five of the patients (50%) underwent chemotherapeutic administration with 5-FU, while the other five (50%) underwent treatment with XEL. Table 1 summarizes the demographics.
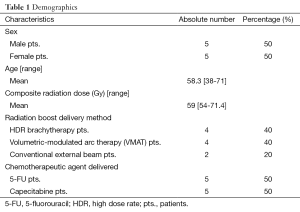
Full table
Eight of the ten patients (80%) who completed NEO successfully proceeded to OLT. Individual etiologies preventing the other two patients (20%) to proceed with OLT included: (I) new diagnosis of metastasis (n=1) and (II) death due to GI bleed (n=1).
Analysis of OS and LC
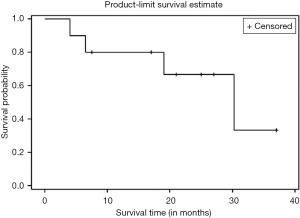
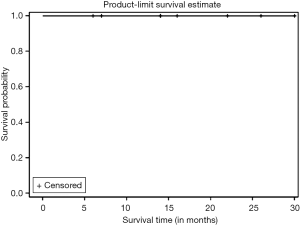
Kaplan Meier AR of OS of all patients who underwent NEO (n=10) as well as those who underwent NEO-OLT (n=8) are illustrated on Figures 1-3 respectively.
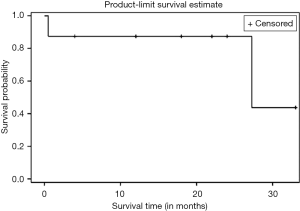
Six, twelve, and twenty-four months OS for those patients who underwent NEO only was 90%, 80%, and 67%, respectively. Median follow-up was 30.25 months with largest follow-up at 37 months. Six, twelve, and twenty-four months OS for patients who had NEO-OLT was 87.5%, 87.5%, and 87.5%, respectively. Median follow-up was 27.25 months with largest follow-up at 33 months.
Kaplan Meier AR of LC of all patients who underwent NEO-OLT is illustrated on Figure 3. Six, twelve, and twenty-four months LC was 100% at each interval. Largest follow-up was at 30 months.
Secondary analysis (including complications)
Thirty-eight percent of patients who underwent NEO-OLT had a pathological complete response (pCR) from surgical specimens analyzed from the OLT procedure. Twenty-five percent of patients required a Whipple procedure after OLT due to positive margins on pathological analysis of tissue analyzed from the OLT procedure.
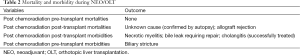
Mortality and morbidity outcomes secondary to NEO or NEO-OLT are illustrated in Table 2.
Full table
Discussion
CCA is a highly aggressive malignancy with features of biliary epithelial differentiation (4,6). While resection is the standard of care, many patients present with unresectable disease (7-10,16,17). However, the results with OLT alone for unresectable disease have been disappointing (11,12). Survival and LC outcomes from previously reported single institution series of NEO-OLT for CCA are significantly better versus resection (14,15,18-22). While our sample size is small, we believe it is imperative to report our series particularly given the surprising scarcity of published data since Rea et al.’s (15) article. Our present study shows an OS CR of 75% at median follow-up of 2.27 years. The 2 months recent Mayo reviews show a decline of ~10% in 5 years OS from their 2005 series (15) to 72% in 2008 (20) followed by 74% in 2012 (21,22). Hence, our result is comparable to Mayo’s series, albeit with a shorter follow-up time and a significantly smaller cohort. Moreover, it is significantly improved from the 45% 5 years OS obtained by Nebraska with median follow up of 7.5 years (14). Furthermore, our TFS as well as local and distant control after OLT remained at 100% at maximum 30 months follow-up. Our TFS was an improvement from Nebraska’s and Mayo’s series [82% with median follow-up of 7.5 years (14) and 92% with median follow-up of 5 years (15), respectively] (Table 3). Among the recurrences noticed in Rea et al. (15) within our study’s median follow-up of 2.27 years included: two patients in patient #1, and chest wall/percutaneous biliary tube site recurrence in patient #2.

Full table
Our data shows that the two mortalities after NEO-OLT were not secondary to local or distant failure. However, one of the mortality etiologies, allograft rejection at 27.25 months, was directly related to the medical management post-OLT. The second patient required a Whipple during the OLT secondary to a bile duct positive margin discovered on pathologic analysis. This patient was found unresponsive at home 2 weeks after the OLT. Detailed analysis of the autopsy showed “infarction of right lobe; IVC thrombosis; 200 cc of serosanguinous ascites, and 150 cc of localized hemorrhage next to the pancreatoduodenectomy site”. The final pathology report of cause of death was documented as “unknown”. Prior series have shown multiple NEO-OLT patients expiring from hepatic artery thrombosis after the OLT, hence it is not implausible that the pathology findings may be indirectly related to her death (15).
Interestingly, other series have discussed the relevant role that a positive margin has due to the potential requirement for a Whipple, which adds to the morbidity/mortality risk. For instance, Duignan et al. (19) showed that 30% of their OLT patients required a Whipple operation. Two of the four deaths in the series had undergone OLT plus Whipple, and two of four other deaths required a re-transplantation. Of our eight NEO-OLT patients, there was only one other patient that had a positive margin requiring a Whipple, and that patient is still alive at 24 months after OLT.
Our 38% pCR rate is reassuring, and comparable with prior series given the relatively wide range published (23-55%) (14,15,18-22). All patients with a pCR are still alive at last follow-up. We agree with Panjala et al. (18) that with progressive improvements in abdominal MRI imaging and MRCP, that we may be able to better delienate the pre NEO gross tumor volume. This is a key issue in this field as prior series have shown that explants reveal tumor dimensions beyond the selection criteria. Hence, this raises the complexity in diagnosis and gross tumor volume design in the setting of either periductal or intraductal infiltrating disease. Fusion of the MRI abdomen to the simulation CT to design the pre NEO GTV is critical and we recommend it in all cases.
There are noticeable differences in the execution of this study as compared to the published series from Mayo (15,20,21). First, four of our patients (40%) received VMAT as the technique of their radiation boost. On the other hand, Mayo used low dose rate (LDR) brachytherapy with Ir192 in their series. Since then, other series have used conventional external radiotherapy as boost (18), however we are the first group to utilize VMAT. While the number of patients in our analysis is inadequate to establish any significant logistic regression analysis linking whether use of VMAT vs. brachytherapy results in improved outcome, our excellent OS and LC rates suggest that, at the very least, use of VMAT is most likely not detrimental. Further research with a bigger cohort of patients evaluating acute and long term toxicity with VMAT dose volume histograms would be very useful.
Second, five patients (50%) received XEL instead of 5-FU. Again, OS and LC actuarial analysis did not show a significant worsening in OS or LC as compared to the other institutional series that have strictly used 5-FU (14,15,20,21). This argues that XEL may possibly be an adequate substitute for 5-FU in the chemotherapy portion of the treatment. Increasing accrual of patients will hopefully help us design logistic regression analysis that will tell us if a particular chemotherapy agent is associated with improved outcomes. Furthermore, given the recent data from the ABC trial in metastatic bile duct or gallbladder cancer showing a OS improvement of 11.7 vs. 8.2 m (P=0.002) with gemcitabine-cisplatin (23), it raises the question whether incorporating these agents in the pre OLT portion of management may be beneficial.
Limitations of our study include the small sample size limiting us at the present time in performing subgroup predictive or prognostic analysis. Further research evaluating factors such as whether pCR, PNI, PSC, or explant tumor size affects OS would be very useful. Furthermore, acute and long term toxicity comparisons between VMAT vs. HDR as the boost technique would also be helpful.
We continue at our institution to perform NEO-OLT and we expect to subsequently publish an updated series with a longer sample size and follow-up. However, given the enormous amount of resources in multidisciplinary management required with this technique in a fairly uncommon condition, we concluded that it is appropriate to report our data at this time.
Conclusions
Our outcomes using NEO-OLT for CCA are promising and comparable to other series. These results further justify (I) use of NEO and (II) prioritization of available transplant livers for CCA management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. an unusual tumor with distinctive clinical and pathological features. Am J Med 1965;38:241-56. [PubMed]
- Launois B, Campion JP, Brissot P, et al. Carcinoma of the hepatic hilus. Surgical management and the case for resection. Ann Surg 1979;190:151-7. [PubMed]
- Parker SL, Tong T, Bolden S, et al. Cancer statistics, 1996. CA Cancer J Clin 1996;46:5-27. [PubMed]
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [PubMed]
- Hirohashi K, Uenishi T, Kubo S, et al. Macroscopic types of intrahepatic cholangiocarcinoma: clinicopathologic features and surgical outcomes. Hepatogastroenterology 2002;49:326-9. [PubMed]
- Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology 2004;66:167-79. [PubMed]
- Burke EC, Jarnagin WR, Hochwald SN, et al. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg 1998;228:385-94. [PubMed]
- Pandey D, Lee KH, Tan KC. The role of liver transplantation for hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2007;6:248-53. [PubMed]
- van der Gaag NA, Kloek JJ, de Bakker JK, et al. Survival analysis and prognostic nomogram for patients undergoing resection of extrahepatic cholangiocarcinoma. Ann Oncol 2012;23:2642-9. [PubMed]
- Silva MA, Tekin K, Aytekin F, et al. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol 2005;31:533-9. [PubMed]
- Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 2000;69:1633-7. [PubMed]
- Robles R, Figueras J, Turrión VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg 2004;239:265-71. [PubMed]
- Foo ML, Gunderson LL, Bender CE, et al. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys 1997;39:929-35. [PubMed]
- Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant 2002;2:774-9. [PubMed]
- Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451-8; discussion 458-61. [PubMed]
- Casavilla FA, Marsh JW, Iwatsuki S, et al. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg 1997;185:429-36. [PubMed]
- Lieser MJ, Barry MK, Rowland C, et al. Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg 1998;5:41-7. [PubMed]
- Panjala C, Nguyen JH, Al-Hajjaj AN, et al. Impact of neoadjuvant chemoradiation on the tumor burden before liver transplantation for unresectable cholangiocarcinoma. Liver Transpl 2012;18:594-601. [PubMed]
- Duignan S, Maguire D, Ravichand CS, et al. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: a single-centre national experience. HPB (Oxford) 2014;16:91-8. [PubMed]
- Rosen CB, Heimbach JK, Gores GJ. Surgery for cholangiocarcinoma: the role of liver transplantation. HPB (Oxford) 2008;10:186-9. [PubMed]
- Rosen CB, Darwish Murad S, Heimbach JK, et al. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: is pretreatment pathological confirmation of diagnosis necessary? J Am Coll Surg 2012;215:31-8; discussion 38-40. [PubMed]
- Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98.e3; quiz e14.
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [PubMed]