Socioeconomic status, p53 abnormalities, and colorectal cancer
Introduction
In the United States, colorectal cancer (CRC) is the third most common incident cancer and the third highest cause of cancer death for both men and women (1). When CRC is diagnosed at an early stage, the 5-year relative survival is 90.4%, but for patients with metastatic disease, 5-year survival is only 11.6% (2). Factors associated with stage at presentation and survival include race (3), gender (3), socioeconomic status (SES) (4,5), and molecular abnormalities, such as abnormal expression of p53 (6). Although SES has emerged as an important factor relating to CRC, the mechanism through which it affects survival has not been elucidated. There is a need to identify genetic factors that contribute to SES differences and to progression of CRCs.
Genetic alterations, including those in the p53 gene, occur during the development of CRCs (7). Patients whose CRCs exhibit nuclear accumulation of p53 (p53nac) have decreased survival after resection, and p53nac may be a useful prognostic factor (8,9). Although patient race/ethnicity and SES are related, they do not measure the same construct (10). Therefore, it is important to determine if SES and race are associated with p53nac. Environmental factors associated with SES may be related to the genetic alterations in carcinogenesis. For women with breast cancer, p53 mutations were associated with SES and may have an association with the poorer prognosis of women of low SES (11). Thus, there is a need to identify the genetic factors that contribute to SES differences and progression of CRCs. This study aimed to evaluate the association between measures of SES, including health insurance and employment status, and p53nac among a cohort of patients with CRC.
Patients and methods
Patients
An existing database for a cohort of patients (N=1,135) who received curative or palliative resection for sporadic CRC at the University of Alabama at Birmingham from 1981 to 2002 was utilized for this study. This investigation, which was approved by the Institutional Review Board at the University of Alabama at Birmingham, did not require informed consent. The data were obtained from medical records, physician charts, and surgical pathology and radiology reports. For a subset of patients, archival tissues were obtained and evaluated for p53nac status (N=590). Only patients with data on both SES and p53nac status were included for this analysis (N=249).
Measures of socioeconomic status proxies
Measures of SES (employment status; and Medicaid, Medicare and/or private insurance coverage) were abstracted from physician charts and medical records. Patients who were specified as “none” for employment were categorized as unemployed and those who gave any other response (including “unknown”) as employed. Because people under the age of 65 typically receive Medicare benefits only if they have a disability or end-stage disease, a dichotomous variable for Medicare status was created as a proxy for disability for such patients. Medicare coverage was not included among all age groups; it is typically available irrespective of SES after the age of 65. Information on insurance coverage was categorized as ‘yes’ or ‘no’ for Medicaid, Medicare due to disability, and private insurance.
Evaluation of nuclear accumulation of p53
For a series of consecutive CRC patients, the phenotypic expression of p53 (p53nac) in CRCs was determined by immunohistochemistry (IHC). As described previously (8,9), only tumor cells with distinct nuclear immunostaining for p53nac were considered positive, and the tumor was considered positive only if p53nac was identified in 10% or more of all malignant cells in a tissue section. The cut-off value of 10% positivity was chosen because it showed the highest concordance between p53nac and point mutations of the p53 gene, as detected by single-strand conformational polymorphism analysis (95% of point mutations) (12).
Other covariates of interest
Due to the small number of patients, only major prognostic factors (age, sex, race, and tumor stage) were included. Age at the time of surgery was included as a continuous variable (range, 26-93 years). Patients were categorized as white (non-Hispanic Caucasian) or black (non-Hispanic African-American) based on the race listed in the medical record. Tumor stage was categorized using the TNM system as Stages I, II, III, or IV according to the criteria of the American Joint Committee on Cancer (13).
Statistical analysis
Descriptive statistics were presented according to p53 status. Chi-square tests for categorical variables and t-tests for continuous variables were used to compare demographic and clinical characteristics. Logistic regression was used to calculate odds ratios (OR) along with 95% confidence intervals (CI) for the association between measures of SES and p53 status. Unadjusted models and models adjusted for all covariates of interest were developed. A two-sided probability of 0.05 was considered statistically significant.
Results
Tumors from 140 patients (56.2%) had p53nac, and tumors from 109 patients (43.8%) had native p53. Patients with p53nac were marginally older, tended to have late stage disease (Stage III/IV), were less likely to be unemployed, and were more likely to have Medicaid coverage (Table 1). Patients who were unemployed were more likely to be female (70.7% versus 48.9%) and older (69.8 versus 64.4 years old) (data not shown). Patients with Medicaid coverage had a higher proportion of females (82.8% versus 55.9%) and were more likely to be black (75.9% versus 44.6%) (data not shown). Patients with private insurance had a significantly lower proportion of females (53.7% versus 69.0%) and were more likely to be white (64.8% versus 27.6%) (data not shown). There were no statistical differences between patients under the age of 65 years who had Medicare (disabled) and those who did not have Medicare (data not shown).
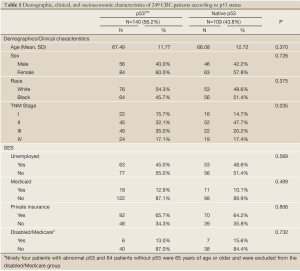
Full table
As shown in Table 2, in unadjusted analyses, the odds of having p53nac for unemployed patients were 0.86 relative to employed patients (95% CI =0.52, 1.43). For patients with Medicaid coverage, the odds of having p53nac were 1.31 times higher than for patients without Medicaid (95% CI, 0.59, 2.91). No association was seen between private insurance coverage and p53nac prevalence (OR 0.94, 95% CI, 0.55, 1.58). Among patients under the age of 65, those with Medicare had 0.81 times the odds of having p53nac compared to patients without Medicare (95% CI, 0.25, 2.64). After adjustment for age, sex, race and tumor stage, all ORs drew marginally closer to the null, except for the association with unemployment, which moved farther from the null (unadjusted OR 0.86 versus adjusted OR 0.74).
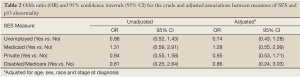
Full table
Discussion
Although the unadjusted and adjusted estimates for the association between the measures of SES with p53nac were not statistically significant, a weak association was detected among Medicaid recipients. Patients of low SES may experience different exposures (e.g., diet, infections, air quality, and other environmental exposures) that lead to abnormal p53. Patients with Medicaid coverage may be most representative of low SES patients since Medicaid is typically provided only to low income individuals and families. For patients with Medicaid coverage, the odds of having p53nac were 1.28 times greater than for patients without Medicaid. This positive association supports our hypothesis that low SES patients have higher odds of p53 abnormalities. The finding, however, was not statistically significant.
The other potential measures of SES did not support our hypothesis, but this may be due to limitations in obtaining SES information from medical records. Information on employment was available only in the medical records of individuals, and, since a higher proportion of patients considered unemployed were females and older, these patients may have either had an employed spouse or have been retired and receiving retirement benefits. Therefore, unemployment as measured in this study may not have been a reliable indicator of low SES. For private insurance, actual rates of coverage vary substantially across plans, with variations in both employer and employee premium contributions and in cost-sharing amounts (14). Therefore, having private health insurance coverage may not directly correlate with SES, as patients could be underinsured or unable to pay the cost-sharing for some procedures. Similarly, patients who receive Medicare prior to retirement were likely previously employed, so it is possible that this group was not of low SES prior to the disability and therefore did not experience the same past exposures as did other low SES patients.
In a previous study, breast cancer patients with low SES (deprivation category 10) had 4.63 times the odds of a p53 mutation compared to patients with high SES (deprivation category 1-9), without adjustment for other factors (11). Low SES breast cancer patients with p53 mutations had poorer survival than other women. After adjustment for potential confounders, these patients had 2.52 times the rate of death than other breast cancer patients. The association found in this study between Medicaid and p53nac did not have the same magnitude as reported in the breast cancer study (11). This difference could be due to an inherently weaker relationship between SES and p53nac among CRC patients compared to breast cancer patients, to imprecision in our estimate due to limited sample size, or to regional differences in SES and/or p53nac.
Most of the alterations in the p53 gene are point (missense) mutations, which lead to altered forms of the p53 protein. These mutant forms generally have a longer half-life than native (wild-type, wt) p53 and can be detected by routine IHC. p53 nuclear accumulation (p53nac) is not necessarily due to p53 gene mutations, it may also be due to formation of complexes between wt-p53 and other nuclear proteins (e.g., the large T antigen), viral proteins (e.g., SV40), or the major heat shock proteins (hsc-70, 72, and 73) (15). Such complexes could be the basis for the existence of nonfunctional p53 (16). In our earlier studies (12,17), without use of an antigen recovery (AR) procedure (boiling the tissues in microwave), we demonstrated that, for CRCs (n=107), the IHC technique identified 95% of missense point mutations in p53, using a 10% staining cutoff for p53nac. When this cut-off value was used, <10% of CRCs exhibited p53nac without a point mutation in the p53 gene (12). Furthermore, p53nac was used to assess the prognoses for CRC patients (8,9,18). Since the data presented in current study were generated following the above described conditions, p53nac is likely to represent underlying p53 gene mutations and suggests a nonfunctional status of p53. Moreover, detection of abnormal p53 by IHC is a simple and cheap technique to use in clinical settings.
Limitations of this pilot study include that there was not sufficient statistical power to detect modest associations between SES and p53nac. Further, although health insurance information was available from medical records, information on other commonly used measures of SES, such as education and income, was not available. Employment status may not accurately represent a patient’s SES, since information on household income or spouse’s employment was not included in the medical record. Finally, both race and SES have effects on incidence and mortality from CRC (4,19), but, due to our small sample size, we were unable to assess an interaction or effect of race on the association between SES and p53nac.
Despite these limitations, this study is, to our knowledge, the first to investigate the association between SES and p53 status among CRC patients. The possible association found between low SES and p53nac in CRC patients was not as strong as was found for breast cancer patients (11). Future studies should focus on the association between income and education as markers of SES with p53nac and should investigate possible interaction between race and SES. It may be important to determine what exposures related to SES cause abnormalities in p53. Although a small fraction of low SES patients had a higher proportion of p53nac, our findings suggest that it is important to identify the factors that cause molecular abnormalities (like p53nac) in relation to SES factors and to evaluate their role in CRC development and progression. Furthermore, similar studies will aid in understanding the molecular pathobiology of malignancies and in identifying susceptible individuals within high-risk populations.
Acknowledgements
This work was supported by grants from the National Institutes of Health [2U54-CA118948, R01-CA98932, R03-CA139629 to UM]; and a National Cancer Institute Cancer Training Grant [5R25-CA047888 to EV].
In 2011, the results were presented as a poster at the 102nd Annual Meeting of the American Association for Cancer Research in Orlando, Florida. Also, we thank Dr. Donald Hill for his critical review of this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- American Cancer Society. Facts and Figures 2011-2013. Atlanta, GA, 2011:1-64.
- Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2007. Bethesda, MD. National Cancer Institute, 2009.
- Paquette I, Finlayson SR. Rural versus urban colorectal and lung cancer patients: differences in stage at presentation. J Am Coll Surg 2007;205:636-41.
- Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer 2007;110:660-9.
- Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer 2006;107:1189-95.
- Goh HS, Yao J, Smith DR. p53 point mutation and survival in colorectal cancer patients. Cancer research 1995;55:5217-21.
- Hollstein M, Sidransky D, Vogelstein B, et al. p53 mutations in human cancers. Science 1991;253:49-53.
- Manne U, Myers RB, Moron C, et al. Prognostic significance of Bcl-2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. Int J Cancer 1997;74:346-58.
- Manne U, Weiss HL, Myers RB, et al. Nuclear accumulation of p53 in colorectal adenocarcinoma: prognostic importance differs with race and location of the tumor. Cancer 1998;83:2456-67.
- Williams DR, Mohammed SA, Leavell J, et al. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci 2010;1186:69-101.
- Baker L, Quinlan PR, Patten N, et al. p53 mutation, deprivation and poor prognosis in primary breast cancer. Br J Cancer 2010;102:719-26.
- Grizzle WE, Myers RB, Manne U, et al. Immunohistochemical evaluation of biomarkers in prostatic and colorectal neoplasia. In: Hanausek M, Walaszek Z, eds. John Walker’s Methods in Molecular Medicine-Tumor Marker Protocols. Totowa, NJ: Humana Press, 1998:143-60.
- Edge SB, Byrd DR, Compton CC, et al. eds. American Joint Committee on Cancer, American Cancer Society: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag, 2010.
- Claxton G, DiJulio B, Whitmore H, et al. Health benefits in 2010: premiums rise modestly, workers pay more toward coverage. Health Aff (Millwood) 2010;29:1942-50.
- Shaulsky G, Goldfinger N, Ben-Ze’ev A, et al. Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol Cell Biol 1990;10:6565-77.
- Jia L, Liu Y, Yi X, et al. Endometrial glandular dysplasia with frequent p53 gene mutation: a genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clin Cancer Res 2008;14:2263-9.
- Grizzle WE, Myers RB, Manne U, et al. Factors affecting immunohistochemical evaluation of biomarker expression in neoplasia. In: Hanausek M, Walaszek Z, editors. John Walker’s Methods in Molecular Medicine - Tumor Marker Protocols. Totowa, NJ: Humana Press, 1998:161-79.
- Manne U. Understanding racial differences in colorectal cancer aids in individualized medicine. Future Oncol 2007;3:235-41.
- Krieger N, Quesenberry C Jr, Peng T, et al. Social class, race/ethnicity, and incidence of breast, cervix, colon, lung, and prostate cancer among Asian, Black, Hispanic, and White residents of the San Francisco Bay Area, 1988-92 (United States). Cancer Causes Control 1999;10:525-37.


