A potential association between exposure to hepatitis B virus and small bowel adenocarcinoma
Introduction
The small intestine comprises 75% of the length of the gastrointestinal (GI) tract, yet small bowel cancers account for only 3 to 6 percent of all GI tract cancers (1). Although overall prevalence rates remain low, incidence has been steadily increasing over the last few years. In 2014, there were an estimated 9,160 new cases and 1,210 deaths secondary to small bowel malignancies (2). The most common small intestinal cancer is adenocarcinoma, which can arise from the duodenum to the ileum (3). Reported risk factors for small bowel adenocarcinoma include celiac disease (4), Crohn’s disease, and inherited adenomatous and polyposis syndromes including familial adenomatous polyposis (FAP), hereditary nonpolyposis colorectal cancer (HNPCC) and Peutz-Jegher syndrome (3,5-9). Despite some positive results of individual studies, systematic review and meta-analysis have not demonstrated tobacco use or alcohol as risk factors for small bowel adenocarcinoma (10).
Diagnosis frequently is delayed since patients may be asymptomatic or have nonspecific symptoms (1). In certain cases, patients may present with symptoms of obstruction or intussusception.
Chronic infection with hepatitis B virus (HBV) has never been described as a risk factor for, or in association with small bowel adenocarcinoma, although infection with the virus is a known risk factor for hepatocellular carcinoma and is increasingly recognized as a potential risk factor for non-Hodgkin lymphoma (11). It is also associated with intrahepatic cholangiocarcinoma carcinoma (ICC) (12). In this paper, we describe an increased prevalence of exposure to HBV in cases of small bowel cancer, and hypothesize a potential mechanism for this finding.
Methods
From May 2009 to December 2014, we implemented an institution-wide screening program for HBV prior to starting chemotherapy. Hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc) were checked prior to first chemotherapy administration; if either serology were positive, a polymerase chain reaction (PCR) for HBV DNA was reflexively performed. Chronic HBV was defined by the presence of HBsAg with or without anti-HBc. Exposure was defined as anti-HBc positivity in the absence of HBsAg positivity.
Patients were considered for inclusion in the present study if they had a documented small bowel cancer as well as positive HBV serologies as defined above. Duodenal, jejunal and ileal adenocarcinomas were included; ampullary (pancreatic type) and appendiceal cancers were excluded. The charts of this subset of patients were further reviewed to determine patient demographic characteristics as well as possible risk factors for small bowel cancer.
The institutional review board approved this study. Descriptive statistics (median, mean, range) were used to describe the study cohort.
Results
Of the 21,611 patients who were screened during the study period, 183 (0.8%) patients were found to be HBsAg positive and 1,924 (8.9%) were found to be HBsAg negative and anti-HBc positive.
Among patients with GI cancers, 5,253 patients were screened. The prevalence of chronic infection was 1.2% (n=63) and prevalence of exposure was 10.8% (n=568) (Table 1). When stratified by specific cancer type, the percentage of HBsAg positivity was highest in patients with hepatocellular carcinoma (10.6%). Evidence of exposure was also highest in patients with hepatocellular carcinoma (21.1%), followed by small bowel cancer (12.5%). There were no HBsAg+ cases of small bowel adenocarcinoma.
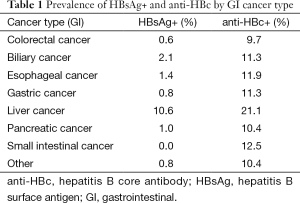
Full table
During the time of the study, 66 new patients were diagnosed with small intestinal cancer. Fifty-six of these cases were screened and seven (12.5%) were found to be HBsAg−/anti-HBc+.
The median age was 56 (range, 51-76) years old. Five patients were male and two were female. Four patients were born outside of the United States (Table 2). Four patients were white, two were black, and one was Asian. Two patients had a history of current or former smoking, and three patients had a history of current or former alcohol use. One patient had a diagnosis of HNPCC. The remainder of patients had no genetic syndromes, history of inflammatory bowel disease or other chronic inflammatory syndromes that could explain their risk.

Full table
Discussion
Our review of institution-wide screening data revealed a relatively high percentage of patients with small bowel cancer exposed to HBV when compared with all GI tract malignancies and when compared to our overall data. This has, to our knowledge never been described.
HBV infection has never been described as a risk factor or in association with small bowel malignancy. Nevertheless, we found a significantly higher rate of anti-HBc positivity in these patients compared to those with biliary cancer, colorectal cancer, gastric cancer, and pancreatic cancer. The overall age-adjusted prevalence of HBsAg and anti-HBc between 1999 and 2006 in the United States has been measured to be 0.27% and 4.7% respectively (13). In our study population of patients with small bowel cancer, the prevalence of anti-HBc was 12.5%. We reviewed seven cases of small bowel adenocarcinoma for potential risk factors and found only one case (Patient 2) that could be explained by known risk factors (HNPCC). To our knowledge this is the first report to examine a possible association between HBV exposure and risk for small bowel adenocarcinoma.
Such an association is plausible. HBV induces malignant transformation of hepatocytes to develop HCC (12). Similarly, in cases of cholangiocarcinoma, it has been postulated that HBV viral nucleic acids and proteins, specifically HBx protein, can induce ICC (14,15). HBx protein has been shown to activate expression of human telomerase reverse transcriptase, leading to tumorigenesis in cholangiocytes (12,16).
HBsAg has been detected in pure bile in patients with acute and chronic infections (17). In the presence of damage to the intestinal mucosa, exposure to bile acids may induce dysplastic changes. Bile acids were initially proposed as carcinogenic; however later studies showed that they increase tumorigenesis by carcinogens, of which HBV is a known example. Bile acids induce DNA damage through the induction of reactive oxygen species (18). Therefore, adenocarcinoma in the duodenum seen after cholecystectomy has been attributed to proximity to the common bile duct (19). The most common location of small bowel adenocarcinomas is in the ampullary region of the duodenum (D2) (20). This also logically follows from the flow of bile through the common bile duct as it enters into the duodenum at D2.
Our study has significant limitations. Our data was obtained as part of a larger data acquisition, which can be misleading. The overall number of patients with small bowel adenocarcinoma is low, and we recognize that the increased percentage of anti-HBc in this population may not persist with further prospective data collection. In addition, we acknowledge that our hypothesis would be strengthened had we found a higher rate of HBsAg+ in small bowel cancer cases. Due to the rarity of these cases (66 cases over a 5-year period), and our overall low HBsAg prevalence (0.8%), it is not surprising that we could not detect this.
Since small bowel cancers are often diagnosed at a later stage when surgical cure is no longer an option, identifying at-risk patients could be crucial to earlier diagnosis and improved outcomes. This association warrants further study, both with larger numbers of patients, perhaps across institutions. In addition, staining small intestinal tumors for hepatitis B core antigen would be of interest, as positive findings would support our proposed mechanism and lend support to further investigation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Williamson JM, Williamson RC. Small bowel tumors: pathology and management. J Med Assoc Thai 2014;97:126-37. [PubMed]
- Shenoy S. Primary small-bowel malignancy: update in tumor biology, markers, and management strategies. J Gastrointest Cancer 2014;45:421-30. [Crossref] [PubMed]
- Ludwig E, Kurtz RC. Tumors of the Small Intestine. In: Sleisenger MH, Feldman M, Friedman LS, et al., editors. Sleisenger and Fordtran’s gastrointestinal and liver disease: pathophysiology, diagnosis, management. Philadelphia, PA: Saunders/Elsevier, 2010.
- Bettini AC, Beretta GD, Sironi P, et al. Chemotherapy in small bowel adenocarcinoma associated with celiac disease: a report of three cases. Tumori 2003;89:193-5. [PubMed]
- Koornstra JJ. Small bowel endoscopy in familial adenomatous polyposis and Lynch syndrome. Best Pract Res Clin Gastroenterol 2012;26:359-68. [Crossref] [PubMed]
- Schulmann K, Brasch FE, Kunstmann E, et al. HNPCC-associated small bowel cancer: clinical and molecular characteristics. Gastroenterology 2005;128:590-9. [Crossref] [PubMed]
- Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis 2014;46:97-104. [Crossref] [PubMed]
- Cahill C, Gordon PH, Petrucci A, et al. Small bowel adenocarcinoma and Crohn's disease: any further ahead than 50 years ago? World J Gastroenterol 2014;20:11486-95. [Crossref] [PubMed]
- Wangler MF, Chavan R, Hicks MJ, et al. Unusually early presentation of small-bowel adenocarcinoma in a patient with Peutz-Jeghers syndrome. J Pediatr Hematol Oncol 2013;35:323-8. [Crossref] [PubMed]
- Bennett CM, Coleman HG, Veal PG, et al. Lifestyle factors and small intestine adenocarcinoma risk: A systematic review and meta-analysis. Cancer Epidemiol 2015;39:265-73. [Crossref] [PubMed]
- Fedewa SA, Sauer AG, Siegel RL, et al. Prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol Biomarkers Prev 2015;24:637-52. [Crossref] [PubMed]
- Li M, Li J, Li P, et al. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol 2012;27:1561-8. [Crossref] [PubMed]
- Wasley A, Kruszon-Moran D, Kuhnert W, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis 2010;202:192-201. [Crossref] [PubMed]
- Wang WL, Gu GY, Hu M. Expression and significance of HBV genes and their antigens in human primary intrahepatic cholangiocarcinoma. World J Gastroenterol 1998;4:392-396. [Crossref] [PubMed]
- Perumal V, Wang J, Thuluvath P, et al. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol 2006;37:1211-6. [Crossref] [PubMed]
- Zou SQ, Qu ZL, Li ZF, et al. Hepatitis B virus X gene induces human telomerase reverse transcriptase mRNA expression in cultured normal human cholangiocytes. World J Gastroenterol 2004;10:2259-62. [PubMed]
- Hoefs JC, Renner IG, Askhcavai M, et al. Hepatitis B surface antigen in pancreatic and biliary secretions. Gastroenterology 1980;79:191-4. [PubMed]
- Theisen J, Peters JH, Fein M, et al. The mutagenic potential of duodenoesophageal reflux. Ann Surg 2005;241:63-8. [PubMed]
- Coats M, Shimi SM. Cholecystectomy and the risk of alimentary tract cancers: a systematic review. World J Gastroenterol 2015;21:3679-93. [Crossref] [PubMed]
- Goldner B, Stabile BE. Duodenal adenocarcinoma: why the extreme rarity of duodenal bulb primary tumors? Am Surg 2014;80:956-9. [PubMed]


