Regorafenib could cause sinusoidal obstruction syndrome
Introduction
Regorafenib, a broad-spectrum tyrosine kinase inhibitor, has recently been approved worldwide as a drug for patients with advanced or metastatic colorectal cancer. Its positive effect has been proven, but there are warnings that regorafenib could be associated with a serious adverse effect: It could cause fatal acute liver failure. Despite emphasis on the side effect in its drug information insert, the mechanism by which regorafenib causes liver failure has not been elucidated.
In this report, we describe a patient who experienced acute liver failure caused by sinusoidal obstruction syndrome (SOS). Previously described as veno-occlusive disease, SOS is a common hepatic complication following bone marrow transplantation (1). Chemotherapy for solid tumors seldom induces SOS, and it is believed that SOS-related conventional chemotherapy is generally less severe than that following bone marrow transplantation (2). The U.S. Food and Drug Administration (FDA), however, has warned that regorafenib could induce severe, sometimes fatal hepatotoxicity. It is unclear how often regorafenib-induced liver failure is associated with SOS, but our report should provide a better understanding of this adverse effect.
Case presentation
A 74-year-old man was admitted to our hospital with appetite loss, jaundice, and abdominal fullness with ascites. At 17 months before admission to our hospital, he was diagnosed as having advanced sigmoid colon cancer with peritoneal dissemination at another institution. After construction of an artificial anus, he underwent combination chemotherapy that included oxaliplatin, TS-1, and cetuximab for 4 weeks, but it failed to prevent disease progression. Subsequent regimens (e.g., irinotecan/TS-1/bevacizumab, panitumumab, and bevacizumab/capecitabine/irinotecan) were also ceased because of their insufficient effect. He was then given regorafenib at 120 mg/day for 4 weeks. He experienced appetite loss and diarrhea starting 2 weeks after beginning regorafenib, but he continued taking the drug. Because the symptoms continued, a blood examination was performed 7 days before admission to our hospital. The tests revealed his hypovolemic status and the elevated levels of bilirubin and hepatic leakage enzymes. His hepatic function continued to deteriorate despite treatment for hypovolemia, and he was referred to our hospital.
On admission, blood tests revealed an increase in aspartate aminotransferase (AST) to 1,663 IU/L, alanine transaminase (ALT) to 978 IU/L, lactate dehydrogenase (LDH) to 1,052 IU/L, total bilirubin to 2.3 mg/dL, and alkaline phosphatase to 673 IU/L. The albumin level (2.5 g/dL) and platelet count (2.9×104/µL) were decreased. Coagulation tests showed an increase in fibrinogen degradation products (FDP) to 21.2 µg/mL and D-dimer to 9.1 µg/mL. The prothrombin time-international normalized ratio (PT-INR) was prolonged (1.51).
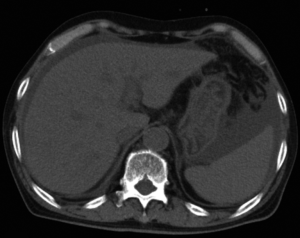
Computed tomography (CT) and ultrasonography (US) showed that the liver was not atrophic or nodular (Figure 1), and the parenchyma was homogeneous. Moderate ascites was observed, and Doppler US revealed extremely decreased portal flow. The portal flow of the right lobe was measured at the umbilical portion and that of the left lobe at the left main trunk. Portal flow in the right lobe showed a to-and-fro pattern, and the flow in the left lobe had decreased to 232 mL/min. Because such a decrease of portal flow is unusual in virus- or drug-induced acute liver failure, and the possibility of Budd–Chiari syndrome was excluded on enhanced CT scans, we reasoned that his liver failure was caused by SOS.
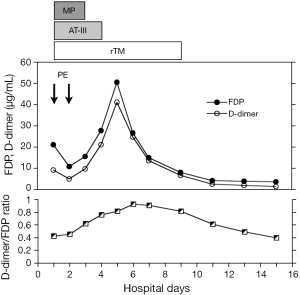
Considering the prolonged prothrombin time, plasma exchange was performed on days 1 and 2. On days 1–3, we administered 1,000 mg of methylprednisolone. In addition, he was given antithrombin III (1,500 IU/day on days 1–4) and recombinant thrombomodulin (380 U/kg/day on days 1–9). The plasma FDP increased until day 5 and then subsequently decreased rapidly. During the anticoagulant treatment, the D-dimer/FDP ratio remained high (Figure 2).
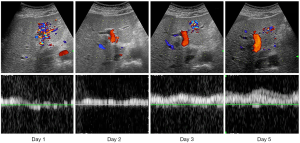
His hepatic function, including ALT, LDH, PT-INR, bilirubin, smoothly improved after plasma exchange. Daily observation with Doppler US revealed that the attenuated portal flow gradually increased, reaching a normal level by day 5 (Figure 3). The ascites diminished day by day and had completely disappeared on day 9.
Discussion
It was an elevated level of LDH over that of ALT on admission that first attracted our attention. LDH is an enzyme essential for catalyzing the conversion of pyruvate, and its transcription increases under hypoxic conditions. A dramatic LDH elevation is believed to be a marker of intrahepatic hypoxia (3). Some patients experiencing acute liver failure caused by hepatitis viruses or drugs show a similar pattern (4). Therefore, such high levels of LDH require a differential diagnosis that includes virus- or drug-induced liver failure, a systemic circulation disorder, Budd-Chiari syndrome, and SOS. Although a rapid diagnosis on admission is not easy, we fortunately had the Doppler US findings in which the portal flow exhibited a to-and-fro pattern, indicating the existence of severe portal hypertension.
In the presence of acute liver failure, the portal flow volume is generally attenuated. An extreme decrease in portal flow, in which Doppler US shows a to-and-fro pattern or hepatofugal flow, is normally not seen before the liver becomes cirrhotic (5-7). In our case, CT and US images showed homogeneous liver parenchyma and no hepatic nodular formation or liver atrophy. The combination of those findings and the portal to-and-fro pattern strongly indicated that there was circulation disturbance in the sinusoids or drainage veins. After excluding Budd-Chiari syndrome and confirming that there was no occlusion in the hepatic vein or inferior vena cava, the most probable diagnosis was SOS.
Although the Doppler US findings seem to be helpful for identifying SOS, the usefulness of the equipment was controversial during the 1990s. Several authors declared that the measurement of portal flow was no use for diagnosing the disease (8,9). During this century, however, results that support the diagnostic usefulness of Doppler US for SOS have been dominant (10,11). Improvement of the equipment could explain the change. Considering the pathogenesis of SOS, the attenuation of portal flow should be observed even in its early stage. Indeed, Myers et al. recently claimed that a number of patients developed SOS without an elevated serum bilirubin level, and they showed reversed portal flow (12).
Anticoagulation and thrombolysis are believed to be reasonable measures to prevent the development of SOS (13). Although defibrotide has shown the most promising results, it has not yet been approved in Japan. Tissue-plasminogen activator has been reported to be ineffective. Therefore, we used AT-III and recombinant thrombomodulin from the day of admission. As shown in Figure 3, plasma FDPs increased after administration of these drugs and abruptly decreased as liver function improved. Such transition seems to reflect the thrombolysis of deposited fibrin and its disappearance. It is noteworthy that the D-dimer/FDP ratio remained high while the FDP level was elevated, which indicated that the treatment not only induced thrombolysis but also might have prevented new fibrin deposition.
Although the FDA reported that severe regorafenib-induced liver injury with fatal outcome occurred in 0.3% of those taking the drug, the mechanism of hepatotoxicity remains unclear. Akamine et al. recently reported a patient with acute liver failure after administration of regorafenib. They observed the portal to-and-fro pattern and dramatic elevation of serum LDH, which suggested that the liver failure could be caused by SOS (13). The rare occasion of liver failure caused by regorafenib seems to open a possibility that a condition other than intake of the drug might be required to develop severe liver injury. In our case, the patient experienced diarrhea around the onset of the liver injury, which implies that decreased hepatic blood flow caused by hypovolemia could trigger fibrin deposition in the hepatic microcirculation. However, it is still impossible to grasp an overview of the mechanism of liver failure caused by regorafenib because reports describing the details of the clinical course have been scarce. We need to accumulate and analyze the patients with regorafenib-induced liver failure.
We described herein a patient with acute liver failure caused by regorafenib. We concluded that the Doppler US results indicated that the liver failure was caused by SOS. Markedly elevated serum LDH levels were also useful for identifying a hypoxic situation in the liver. The FDA recommends monitoring serum ALT, AST, and bilirubin to detect liver failure in its early stage. We believe that LDH should be added to that list.
Acknowledgements
The authors would like to thank Ms. Keiko Nishimura for editorial support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol 2014;4:332-46. [Crossref] [PubMed]
- Cefalo MG, Maurizi P, Arlotta A, et al. Hepatic veno-occlusive disease: a chemotherapy-related toxicity in children with malignancies. Paediatr Drugs 2010;12:277-84. [Crossref] [PubMed]
- Firth JD, Ebert BL, Pugh CW, et al. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3’ enhancer. Proc Natl Acad Sci U S A 1994;91:6496-500. [Crossref] [PubMed]
- Kotoh K, Kato M, Kohjima M, et al. Lactate dehydrogenase production in hepatocytes is increased at an early stage of acute liver failure. Exp Ther Med 2011;2:195-9. [PubMed]
- Yang SS, Wu CH, Chen TK, et al. Portal blood flow in acute hepatitis with and without ascites: a non-invasive measurement using an ultrasonic Doppler. J Gastroenterol Hepatol 1995;10:36-41. [Crossref] [PubMed]
- Tai D, Changchien CS, Chen CJ, et al. Sequential evaluation of portal venous hemodynamics by Doppler ultrasound in patients with severe acute hepatitis. Am J Gastroenterol 1996;91:545-50. [PubMed]
- Deasy NP, Wendon J, Meire HB, et al. The value of serial Doppler ultrasound as a predictor of clinical outcome and the need for transplantation in fulminant and severe acute liver failure. Br J Radiol 1999;72:134-43. [Crossref] [PubMed]
- Hommeyer SC, Teefey SA, Jacobson AF, et al. Venocclusive disease of the liver: prospective study of US evaluation. Radiology 1992;184:683-6. [Crossref] [PubMed]
- Teefey SA, Brink JA, Borson RA, et al. Diagnosis of venoocclusive disease of the liver after bone marrow transplantation: value of duplex sonography. AJR Am J Roentgenol 1995;164:1397-401. [Crossref] [PubMed]
- Yoshimoto K, Yakushiji K, Ijuin H, et al. Colour Doppler ultrasonography of a segmental branch of the portal vein is useful for early diagnosis and monitoring of the therapeutic course of veno-occlusive disease after allogenic haematopoietic stem cell transplantation. Br J Haematol 2001;115:945-8. [Crossref] [PubMed]
- Hashiguchi M, Okamura T, Yoshimoto K, et al. Demonstration of reversed flow in segmental branches of the portal vein with hand-held color Doppler ultrasonography after hematopoietic stem cell transplantation. Bone Marrow Transplant 2005;36:1071-5. [Crossref] [PubMed]
- Myers KC, Dandoy C, El-Bietar J, et al. Veno-occlusive disease of the liver in the absence of elevation in bilirubin in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2015;21:379-81. [Crossref] [PubMed]
- Akamine T, Ando K, Oki E, et al. Acute Liver Failure Due to Regorafenib May Be Caused by Impaired Liver Blood Flow: A Case Report. Anticancer Res 2015;35:4037-41. [PubMed]


