Recognizing the distinct cytomorphologic features of solid pseudopapillary neoplasm of the pancreas
Introduction
Solid pseudopapillary neoplasm (SPN) of the pancreas is an uncommon tumor of uncertain cellular lineage, comprising 0.2-2.7% (1) of exocrine pancreatic neoplasms. Although reported in all ages, SPN is typically encountered in female patients (mean age 25-35 years). The male to female ratio is 1:20 (2). While SPN is slightly more common in the tail, it can occur in any part of the pancreas (3). Approximately one-third of cases are detected incidentally. Some patients may present with abdominal pain. Laboratory tests including serum cancer markers are usually normal. Imaging studies generally reveal a well-circumscribed heterogeneous solid and cystic mass with calcifications. Surgical resection with negative margins is the standard treatment, and is curative in approximately 80% of patients. Prognosis is excellent with an overall five-year survival rate of 95% (3). Although 10-15% of patients may develop metastases (3), [usually liver, regional lymph nodes, mesentery, omentum, or peritoneum (4)], these patients still have a favorable long-term outcome (5).
Case presentation
A 56-year-old male presented to the Emergency Department with a sudden onset of left upper quadrant pain. Blood work including tumor markers CA 19-9 and CEA were within normal limits. Computed tomography of the abdomen revealed a 4.5 cm well-circumscribed focally calcified pancreatic tail mass. Aspiration of the mass showed a limited, sparsely cellular, poorly preserved specimen with a background of hemorrhage, necrosis, and macrophages. A rare thin fibrovascular stalk lined by a multilayer of cuboidal cells with round nuclei and delicate cytoplasm was noted. Rare microacini and single cells were also present. Cell block showed similar findings.
Immunohistochemistry revealed the cells were positive for vimentin, CD10, progesterone, CD56, and nuclear beta-catenin, and negative for synaptophysin, chromogranin, and pankeratin, consistent with a diagnosis of SPN.
The patient underwent distal pancreatectomy and splenectomy. The final pathologic diagnosis was SPN pT2N0, with negative margins. No perineural, lymphovascular, lymph node, or splenic involvement was identified.
Discussion
Aspirations of SPN are usually cellular with small loosely cohesive groups (Figure 1), microacini, and singly dispersed tumor cells. Thin and delicate or thick and hyalinized branching fibrovascular papillae are particularly characteristic of SPN (Figure 2). These vascular stalks are lined by one or more layers of monotonous cuboidal neoplastic cells with indistinct cell borders (Figure 2). The nuclei are uniform, round or ovoid (Figure 3), with finely dispersed chromatin, inconspicuous nucleoli, and occasional intranuclear grooves. The background may show debris, macrophages, necrosis (Figure 1), cholesterol crystals, and hemorrhage. Another very characteristic finding of SPN is metachromatic hyaline globules composed of amorphous myxoid material found intracellularly, extracellularly, or in the microacinar lumina.
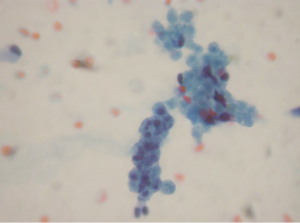
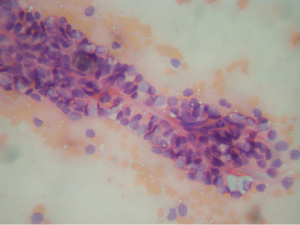
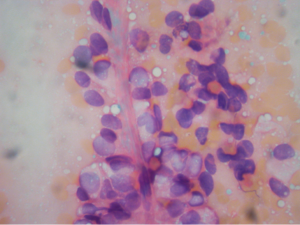
The differential diagnosis of SPN (see summary in Table 1) may include: pseudocyst, pancreatic mucinous neoplasms (PMN), well-differentiated ductal adenocarcinoma (WDDA), pancreatic endocrine neoplasm (PEN), and acinic cell carcinoma (ACC).

Full table
Pseudocysts lack an epithelial lining, resulting in hypocellular smears showing granular debris, hemosiderin, inflammatory cells, and pigmented macrophages. Both SPN and pseudocysts show degenerative changes including necrosis, calcifications, cholesterol crystals, and hemorrhage. However, fibrovascular stalks, papillae, and epithelial cells are not seen in pseudocysts.
Large pools of mucin are characteristic of PMN and should not be seen in SPN. Unlike smears of SPN, aspirations of PMN are paucicellular and show few sheets of columnar mucinous cells with small uniform basally located nuclei, abundant apical cytoplasm, and distinct cell borders. The hypocellularity of the smears, presence of copious thick colloid-type mucin, and columnar morphology should help readily distinguish PMN from SPN.
WDDA may be cytologically bland, but has several distinguishing features. The tumor cells in WDDA are usually seen in large tightly cohesive crowded “drunken honeycomb” sheets and disorderly clusters, rather than the small loosely cohesive non-crowded clusters typical of SPN. WDDA shows scant or abundant vacuolated cytoplasm with intracytoplasmic mucin. Mitoses are a helpful finding since they are infrequent in SPN, but may be identified in WDDA. The nuclei in WDDA show mild anisonucleosis with parachromatin clearing, whereas the nuclei in SPN are monotonous with finely textured evenly dispersed chromatin. Although intranuclear grooves can be seen in both WDDA and SPN, irregular nuclear contours, notches, and convolutions, typical of WDDA, should not be seen in SPN.
PEN can look very similar to the aspiration of SPN. Both neoplasms generally result in cellular aspirates and can show tumor cells in small loosely cohesive clusters. Both can show architectural pattern suggestive of acini. PEN shows rosettes without fibrovascular cores. SPN shows microacinar structures with fibrovascular cores. Although large tissue fragments traversed by delicate capillaries can be seen in PEN, true fibrovascular stalks lined by single or multiple layers of loosely cohesive tumor cells and pseudopapillae should not be seen in PEN. An architectural finding more characteristic of PEN is an abundance of singly dispersed tumor cells and bare tumor nuclei. Prominent population of discohesive tumor cells and naked nuclei are generally not seen in SPN. Both SPN and PEN typically show low-grade cytologic features with small to medium-sized cells, round to oval, uniform nuclei, and indistinct nucleoli. Features more suggestive of PEN include spindle-shaped nuclei and eccentrically placed nuclei, imparting a plasmacytoid appearance. Furthermore, the classic stippled salt-and-pepper chromatin and fine metachromatic cytoplasmic granules are characteristic of PEN. The cells of SPN show evenly distributed finely granular chromatin and pale delicate cytoplasm without significant granularity. While PEN may show focal overt nuclear pleomorphism (endocrine atypia), this finding should not be seen in SPN.
Similar to SPN, ACC also displays cellular aspirates with tumor cells seen in cohesive nests, loose aggregates, and acinar-like arrangements. However, the characteristic pseudopapillary structures and fibrovascular stalks seen in SPN should not be seen in ACC. An architectural finding very characteristic of ACC is numerous single polygonal tumor cells. The tumor cells have eccentrically placed nuclei and prominent nucleoli, as opposed to the centrally placed nuclei with inconspicuous nucleoli typical of SPN. Neither tumor should show prominent nuclear membrane irregularities or significantly coarse chromatin. The cytoplasm in ACC is usually abundant and coarsely granular due to the presence of zymogen granules. Degranulated neoplastic acinic cells, however, may have clear cytoplasm, similar to the pale and delicate cytoplasm of SPN. Lastly, the background of ACC is clean, unlike SPN, which shows degenerative changes and inflammation.
Immunohistochemistry is helpful in distinguishing these entities. SPN stains with vimentin, CD10, progesterone, alpha1 anti-trypsin, and CD56, and exhibits strong nuclear beta-catenin staining (6). It is usually negative for epithelial and neuroendocrine markers, distinguishing it from ACC, WDDA, and PEN.
Histopathologic findings mirror the cytologic features (Figure 4). The vessels coursing through the solid nests of neoplastic cells seen in histology correlate with the fibrovascular stalks of the pseudopapillae seen in the aspirates. In histology, the areas of cellular degeneration caused by discohesion of cells furthest away from the vasculature result in the false papillary architecture as well as the degenerative background seen in the smears.
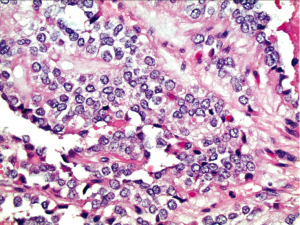
Conclusions
It is important to remember that SPN can occur in men. Aspiration cytology shows distinctive cytomorphology, the most characteristic feature being fibrovascular cores lined by cytologically bland fragile neoplastic cells. Since limited samples are common from aspirations of pancreatic lesions, recognition of this unique feature is of utmost importance in distinguishing SPN from other entities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vassos N, Agaimy A, Klein P, et al. Solid-pseudopapillary neoplasm (SPN) of the pancreas: case series and literature review on an enigmatic entity. Int J Clin Exp Pathol 2013;6:1051-9. [PubMed]
- Bouassida M, Mighri MM, Bacha D, et al. Solid pseudo-papillary neoplasm of the pancreas in an old man: age does not matter. Pan Afr Med J 2012;13:8. [PubMed]
- Yagcı A, Yakan S, Coskun A, et al. Diagnosis and treatment of solid pseudopapillary tumor of the pancreas: experience of one single institution from Turkey. World J Surg Oncol 2013;11:308. [PubMed]
- Guo N, Zhou QB, Chen RF, et al. Diagnosis and surgical treatment of solid pseudopapillary neoplasm of the pancreas: analysis of 24 cases. Can J Surg 2011;54:368-74. [PubMed]
- Tang LH, Aydin H, Brennan MF, et al. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol 2005;29:512-9. [PubMed]
- Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol 2002;160:1361-9. [PubMed]


