Clinically undiagnosed enteropathy associated T-cell lymphoma type II presenting with prolonged lower gastrointestinal tract symptoms: report of an autopsy case and review of diagnostic challenges and clinicopathological correlation
Introduction
In the United States, the small intestine is the fourth most common site involved by extra-nodal lymphomas, after the skin, stomach and oral cavity/pharynx (1). A vast majority of gastrointestinal (GI) tract lymphomas are B-cell lymphomas and the stomach is affected more frequently than all other sites in the GI tract combined (2). T-cell lymphomas are uncommon in the West and T-cell lymphomas affecting the GI tract are rare. A special type of T-cell lymphoma called enteropathy associated T-cell lymphoma (EATL) occurs primarily in the jejunum or ileum and may pose unique diagnostic challenges. Two subtypes of EATL, called EATL-I and EATL-II are recognized based on histology, immunophenotype, and relationship to celiac disease (CD). We present a case of EATL type II in which the diagnosis was not established until autopsy, in spite of extensive endoscopic, clinical laboratory, and radiographic work- up. The relationship between celiac disease and EATL is examined and the practical difficulties to early diagnosis of small intestinal lymphomas and the pathological differential diagnosis are discussed.
Case report
Clinical findings
An 80-year-old woman had a 40-lb weight loss over 10 months followed by diarrhea, nausea, early satiety and fatigue for 3 months. Her past medical history included hypertension, hyperlipidemia and Type II diabetes mellitus, but no history suggestive of chronic inflammatory bowel disease (IBD). At another institution she received an exhaustive workup for infection, IBD and malignancy. Her physical exam was unrevealing. Stool examination was positive for leukocytes, but negative for bacterial pathogens, ova and parasites. Her symptoms did not respond to empiric treatment with levofloxacin and metronidazole. Hemoccult testing was positive and a mild iron deficiency anemia was documented (Hematocrit 33%). She received her first and only upper GI endoscopy after 9 months of weight loss, during which the scope was advanced up to the second part of the duodenum. A Schatski ring identified at the gastro-esophageal junction was ablated. The stomach and duodenum were reportedly normal and no tissue was submitted for pathological examination. Three colonoscopies with biopsies revealed no evidence of malignancy or inflammation, identifying only two small polyps in the ascending colon and diverticuli in the sigmoid colon. She was diagnosed as having a biopsy-negative unspecified colitis and her symptoms improved with subsequent treatment with prednisone and mesalamine. Laboratory testing revealed elevation of carcinoembryonic antigen (3.2 ng/mL) and carbohydrate antigen 19-9 (72 U/mL). No mass lesions were identified on abdominal CT scan and ultrasound and the clinical impression ten days before death was that there was no evidence of malignancy. Three days before her death, her family brought her to the emergency department at our Institution out of concern for her worsening weakness and fears of occult malignancy. Two days later, she was transferred to the medical intensive care unit for respiratory failure and septic shock. She continued to deteriorate and died of cardiac arrest a day later. An autopsy was performed to investigate the cause of death.
Autopsy findings
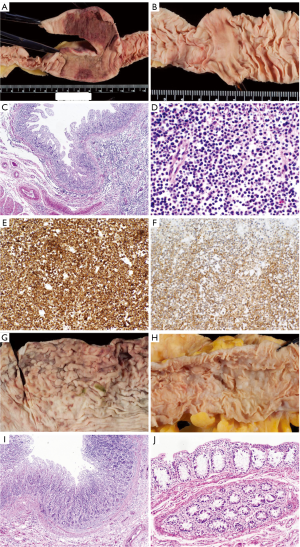
The external examination was significant for stretch marks and excess skin on the flanks and lateral thighs, compatible with the patient’s recent weight loss. Internal examination limited to chest and abdomen showed a 7 cm long segment of thickened jejunum, 204 cm distal to the ligament of Treitz (Figure 1A). It was 7.6 cm in maximum diameter, with an intestinal wall thickened to 0.6 cm (normal adjacent small intestine diameter was 2.8 cm and thickness was 0.4 cm). The mucosal surface was flattened in this and many additional segments from duodenum to ileum (Figure 1B). On histologic examination, the intestinal villi were blunted. Small, uniform lymphocytes without cytological atypia were markedly increased in the lamina propria and epithelium, (Figure 1C,D). They expressed pan-T-cell antigens CD3 (partial), CD7 (Figure 1E), CD8 (partial) and CD56 (Figure 1F) but not CD5. These findings were diagnostic of EATL type II. Gross findings in the stomach were subtle, including thickening of the antral wall and erosions and blunting of the gastric rugae (Figure 1G). Microscopically a diffuse mucosal infiltrate of small lymphocytes was detected (Figure 1I). Lymphoma spared the colon, grossly (Figure 1H) and microscopically (Figure 1J) but involved the liver, pancreas, heart, lungs, adrenal glands and multiple lymph nodes in chest and abdomen (not shown).
Discussion
This patient with several months’ history of weight loss followed by prolonged diarrhea received an exhaustive workup for gastrointestinal malignancy, infection and inflammatory bowel disease. Multiple diagnostic studies were performed, including stool examinations, serologic tests, three lower GI endoscopies with biopsies, radiologic evaluation of the abdomen by computed tomography (CT) and ultrasound. One upper GI endoscopy was performed but no biopsies were taken because a suspicious localized lesion was not seen in the stomach or duodenum. While antibiotic treatments did not offer any relief, steroids controlled her lower GI symptoms to some extent and the clinical impression until a few days before death was that she had a biopsy negative unspecified colitis. Computed tomography (CT) of the abdomen, without enhancement by 18F fluorodeoxyglucose positron emission tomography scan (18F-FDG-PET scan), was not sufficiently sensitive to detect the multifocal involvement by lymphoma in the jejunum and ileum. Malignancy of the small intestine was not suspected or investigated with an effective test, such as double-balloon enteroscopy with appropriate tissue sampling and the diagnosis of EATL was not established until autopsy.
EATL is a rare disease throughout most of the world, with an incidence of 0.5-1 per million per year (3). It accounts for 1.4% of all lymphomas (4) and 5.4% of peripheral T cell lymphomas (3). Two thirds of EATL are Type I, and the other one third are Type II. EATL Type I is more common in Europe and Type II is more common in Asia. Types I and II are equally common in North America. The median age at diagnosis of EATL is approximately 60 years (4). It is more common in men than women: 54-74% of those diagnosed with EATL are men. A history of CD is obtained in half the patients with EATL Type I but only a quarter with EATL Type II (3). Presenting symptoms in both types are abdominal pain (88%), fatigue, nausea/vomiting, anorexia and weight loss (each <40%), and rarely organomegaly. Anemia, elevated lactate dehydrogenase, low albumin and elevated C-reactive protein are common (4). Over 90% EATL arise in the small intestine–most frequently in the jejunum and proximal ileum, and less commonly in large intestine (16%), stomach (8%), lung (5%), skin (5%), bone (3%), liver (2%), spleen (2%) and paranasal sinuses (2%) (3). In most cases the tumor is multifocal with multiple ulcerating raised mucosal masses, but sometimes one or more ulcers or a large exophytic mass (5) or strictures and plaques (6) may be seen.
Stage and tumor size appear to be important prognostic factors (7) and early diagnosis may offer a possibility of cure but remains challenging, as in this case. Consequently, 60% to 70% patients present with advanced disease, most being diagnosed during surgery for acute abdominal complications (4,8). Therefore, in a patient with chronic, intractable GI tract symptoms but with no evidence of gastric or colonic disease on upper and lower endoscopy, diseases of the small intestine (e.g., malignancy, infection, autoimmune disease) should be considered and evaluated. Technics such as video capsule endoscopy, double-balloon enteroscopy, computed tomography (CT) scan combined with 18F fluorodeoxyglucose positron emission tomography (18F-FDG-PET) scan and magnetic resonance enteroclysis are possible modalities to investigate small intestinal malignancy (9).
Prevention of EATL may be feasible in some cases with early diagnosis of CD and adherence to a strict gluten-free diet, which can lead to a four-fold reduction in the risk of EATL in CD, compared to patients who are non-compliant (7). Even compliant CD patients need monitoring for progression to refractory CD (RCD). RCD is divided into two types- type I is characterized by persistent or recurrent symptoms, positive CD-specific serology, and/or villous atrophy after 6-12 months on a gluten free diet and exclusion of other etiologies (10). RCD type II is diagnosed when an abnormal (clonal) population of intraepithelial T-cells is also present. These clonal T-cells show loss of normal surface markers CD3, CD4 and CD8 with preserved expression of intracytoplasmic CD3 in >50% by immunohistochemistry (or >20-25% by flow cytometry). These abnormal T-cells may also be present in lamina propria (11). 60-80% of patients with RCD type II will progress to EATL (12). Endoscopically, RCD type II shows either multiple ulcers (“ulcerative jejunitis”) or large ulcers (>1 cm). The presence of nodules, masses and strictures, as well as cytologic atypia, suggest progression to EATL (10). RCD type II is associated with a 5-year survival rate of only 40-58% (10), but survival may be improved with high-dose chemotherapy and autologous stem-cell transplantation before development of EATL (12).
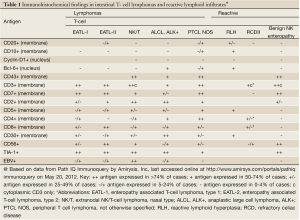
The pathological diagnosis of EATL has potential pitfalls as well. In EATL type I, the lymphocytes are medium-sized to large cells with round or angulated vesicular nuclei, prominent nucleoli and moderate to abundant, pale-staining cytoplasm (5). Less often, the tumor cells are more pleomorphic and sometimes multi-nucleated, resembling anaplastic large cell lymphoma. EATL type I tends to be infiltrated with abundant eosinophils and histiocytes. Coagulative necrosis is common. The intestinal mucosa adjacent to the primary tumor frequently shows enteropathy with villous atrophy, crypt hyperplasia, increased inflammatory cells in the lamina propria, and intraepithelial lymphocytosis (2). In contrast, EATL type II is characterized by multiple foci of small round uniform cells, with dark nuclei and a rim of pale cytoplasm. There is heavy infiltration of the intestinal crypt epithelium, with a few admixed inflammatory cells and no coagulative necrosis. The adjacent intestinal mucosa shows villous atrophy and crypt hyperplasia with marked intraepithelial lymphocytosis involving both crypt and surface epithelium (2). EATL types I and II cannot always be distinguished by morphology, as EATL type I may also present with monomorphic small-medium cells. Immunohistochemical staining is usually helpful and the immunophenotype expected in EATL types and other intestinal T-cell lymphomas are summarized in Table 1. Lymphoma cells stain with CD8 and CD56 in the majority of EATL type II, but only a minority of EATL type I (3). Over 90% of EATL type I are associated with expression of HLA DQ2 or DQ8, versus only 30-40% of EATL type II (5). The differential diagnosis for a tumor of small monotonous lymphocytes in the small intestine also includes certain B-cell lymphomas, primarily mantle cell lymphoma (MCL) (2), but these can be readily distinguished with immunohistochemical staining for pan-B cell antigens such as CD20. Among the other intestinal T-cell lymphomas, NK/T-cell lymphoma, nasal type, may present with small CD56+ lymphocytes, but unlike EATL Type II, these lymphomas are usually negative for CD8 and positive for Epstein Barr virus (13). Anaplastic large cell lymphoma (ALCL) usually consists of large lymphoid cells but rarely the majority of cells may be small to medium sized. The presence of at least a few large anaplastic cells and the characteristic CD30 positive immunostaining reaction are helpful for differentiating these rare lymphomas from EATL type II (14). “NK-cell enteropathy” (15) and the related condition “lymphomatoid gastropathy” (16) are newly described entities in which atypical NK-cells (CD56+) infiltrate one or more GIT sites. Endoscopically there are multiple superficial, discrete, flat or hemorrhagic lesions, or small (<1 cm), patchy, superficial ulcers, as opposed to the nodules, masses and strictures seen in lymphoma. NK-cell enteropathy causes few symptoms and has an uneventful clinical course (15). Finally, reactive lymphoid hyperplasia and CD should also be considered in the differential diagnosis of a dense infiltrate of small monotonous lymphocytes in the mucosa of the small intestine.
Full table
This case highlights the unique diagnostic challenges posed by EATL type II. A high degree of suspicion, use of advanced diagnostic modalities and biopsy of grossly uninvolved site such as stomach may provide the best chance for prompt diagnosis.
Acknowledgements
We wish to thank Kirsten Boland, P.A. (ASCP) for help in autopsy prosection and Susan Reeves and Steven Conlon for expert help with gross and microscopic photography.
Disclosure: The authors declare no conflict of interest.
References
- Wu XC, Andrews P, Chen VW, et al. Incidence of extranodal non-Hodgkin lymphomas among whites, blacks, and Asians/Pacific Islanders in the United States: anatomic site and histology differences. Cancer Epidemiol 2009;33:337-46.
- Burke JS. Lymphoproliferative disorders of the gastrointestinal tract: a review and pragmatic guide to diagnosis. Arch Pathol Lab Med 2011;135:1283-97.
- Delabie J, Holte H, Vose JM, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood 2011;118:148-55.
- Sieniawski MK, Lennard AL. Enteropathy-associated T-cell lymphoma: epidemiology, clinical features, and current treatment strategies. Curr Hematol Malig Rep 2011;6:231-40.
- Issacson PG, Chott A, Ott G, et al. Enteropathy-associated T-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al. eds. Who classification of tumours of haematopoietic and lymhoid tissues. Lyon: International Agency on Research on Cancer, 2008: 289-91.
- Sieniawski M, Angamuthu N, Boyd K, et al. Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood 2010;115:3664-70.
- Chandesris MO, Malamut G, Verkarre V, et al. Enteropathy-associated T-cell lymphoma: a review on clinical presentation, diagnosis, therapeutic strategies and perspectives. Gastroenterol Clin Biol 2010;34:590-605.
- Sun J, Lu Z, Yang D, et al. Primary intestinal T-cell and NK-cell lymphomas: a clinicopathological and molecular study from China focused on type II enteropathy-associated T-cell lymphoma and primary intestinal NK-cell lymphoma. Mod Pathol 2011;24:983-92.
- van de Water JM, Cillessen SA, Visser OJ, et al. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract Res Clin Gastroenterol 2010;24:43-56.
- Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut 2010;59:547-57.
- Verbeek WH, von Blomberg BM, Coupe VM, et al. Aberrant T-lymphocytes in refractory coeliac disease are not strictly confined to a small intestinal intraepithelial localization. Cytometry B Clin Cytom 2009;76:367-74.
- Al-toma A, Visser OJ, van Roessel HM, et al. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood 2007;109:2243-9.
- Ng SB, Lai KW, Murugaya S, et al. Nasal-type extranodal natural killer/T-cell lymphomas: a clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol 2004;17:1097-107.
- Summers TA, Moncur JT. The small cell variant of anaplastic large cell lymphoma. Arch Pathol Lab Med 2010;134:1706-10.
- Mansoor A, Pittaluga S, Beck PL, et al. NK-cell enteropathy: a benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: clinicopathologic features and follow-up in a unique case series. Blood 2011;117:1447-52.
- Takeuchi K, Yokoyama M, Ishizawa S, et al. Lymphomatoid gastropathy: a distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood 2010;116:5631-7.


