Endoscopic versus surgical resection for early colorectal cancer—a systematic review and meta-analysis
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in females and the third in males. A total of 1.2 million new cases and 608,700 deaths are estimated to have occurred in 2008 alone (1). Higher mortality rates are associated with more advanced disease progression (2). As CRC screening continues to gain importance as a prevention tool against advanced disease diagnosis, with direct impact on mortality (3), early disease detection rates rise. Early CRC is defined as invasive neoplasia that does not involve the colonic wall beyond the mucosal and submucosal layers (4) [Tis or T1 according to current American Joint Committee on Cancer TNM classification (2)]. The risk of lymph node metastasis is low following neoplastic invasion of the mucosa, lamina propria, muscularis mucosa, and superficial submucosa of the colorectum, due to the regional absence of a rich lymph vascular network (2,4). Surgical resection has historically been recognized as the gold-standard treatment (either open or laparoscopic assisted), but less invasive techniques [i.e., trans anal endoscopic microsurgery (TEMS), endoscopic mucosectomy resection (EMR), or endoscopic submucosal dissection (ESD)] are emerging as important and safe treatment options (5-7). Nonetheless, there is little data available in the literature concerning the short and long-term outcomes of these new, less invasive treatments, as compared to traditional surgical outcomes. Local expertise of both endoscopic and surgical teams is mainly what determines treatment choice, and the results are often published in the literature with no comparison between techniques (5-7).
The aim of the present study is to acquire and analyze the available data regarding the short- and long-term results of EMR or ESD, when compared with the gold standard surgical (open or laparoscopic) treatment, for the treatment of patients diagnosed with early CRC.
Methods
Registration
This review is registered on PROSPERO international database (from University of York Centre for Reviews and dissemination—www.crd.york.ac.uk/prospero/) under number CRD42014015630.
Eligibility criteria and PICO (Patients, Intervention, Control, and Outcomes)
The search was directed towards comparative studies. No language or publication date filters were applied. The inclusion criteria were:
- Studies including patients with early CRC, defined via post-procedure pathological assessment as malignant adenocarcinoma invading up to the submucosa;
- Studies with the experimental intervention defined as purely endoscopic resections, either EMR or ESD (studies regarding TEMS procedures were not included);
- Studies with the control intervention being surgical treatment, either open or laparoscopic assisted;
- Studies with short and/or long-term results (curative resection rates, en bloc resection rates, procedure times, complications, long-term survival rates).
Abstracts or full texts with data that could not be retrieved were excluded, as well as those with available data that did not discriminate between patients with early or advanced CRC or between those in the control intervention group undergoing colorectal surgery or TEMS.
Data search
Two independent authors performed a systematic review of articles published up to August 2015 in any language, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (8). The searched databases were MEDLINE, EMBASE, LILACS, CENTRAL COCHRANE, and EBSCO. Additional searches were performed on selected studies references, and used the MESH terms reported in the selected studies.
- The MEDLINE search strategy was {(colorectal neoplasms or sigmoid neoplasms or colonic neoplasms) and [(colectomy or colonic surgery or hemicolectomy or sigmoidectomy or rectosigmoidectomy) and (endoscopic resection or endoscopic treatment or ESD or endoscopy or endoscopic submucosal resection)]};
- EMBASE and other databases were searched using the terms ‘Colon cancer’ and ‘surgery’ and ‘endoscopy’.
Study selection
The two reviewers independently assessed eligibility of all screened abstracts according to the inclusion and exclusion criteria discriminated above. Disagreements between the reviewers were resolved by consensus.
Data collection process and outcomes
Only published, available, and comparable data regarding the stated outcomes (i.e., short and long-term results of early CRC treatments) was extracted. The two reviewers independently extracted data directly from the results of each article. The following outcomes were included for data extraction:
- Primary outcomes:
- - En bloc resection: specimen resection and retrieval as a single fragment;
- - Curative (R0) resection: when resection margins were assessed as disease-free on pathological analysis of the surgical specimen, an R0 resection was achieved.
- Secondary outcomes:
- - Complications: all the included studies compared the frequency of complications in absolute numbers for each intervention group. The nature of these complications naturally differed between the two groups: endoscopic complications reported were perforation and post-esd bleeding; surgical complications reported were: wound infection, pelvic abscess, anastomosis leakage, anastomosis bleeding, ileus, peritonitis, diverting stoma, surgical wound dehiscence, surgical wound infection, subcutaneous hematoma, pneumonitis, cholecystitis, abdominal incisional hernia, hives, paroxysmal atrial fibrillation, and delirium;
- - Procedure time: mean duration of the procedures, expressed as means and standard deviations.
Risk of bias in individual studies
The risk of bias in individual studies was assessed using the Newcastle—Ottawa Quality Assessment Scale for Cohort Studies (9). The methods of individual studies were analyzed to search for other possible bias sources.
Summary measures
Individual patient data (expressed as absolute values) were collected for each outcome on each group (endoscopic or surgical treatment) so that the risk difference of that particular outcome between the two groups could be calculated for comparison. 95% confidence intervals (95% CIs) for statistical significance were expressed. NNT and number need to harm (NNH) values were also expressed, whenever statistically significant. Continuous variables had to be stated as means and standard deviations in order to be included in the final analysis. Forest plot graphical expressions were used to demonstrate the relation between sample size and effect size.
Planned methods of analysis
The analysis was performed using the software Review Manager (RevMan) 5.3. (10), obtained from the website Cochrane Informatics & Knowledge Management Department. Risk differences of dichotomous variables were calculated using a fixed effects model, resulting in forest and funnel plots. Mantel-Haenszel tests were employed to calculate a 95% CI for each outcome risk difference; a value of p below 0.05 (95% CI) was considered statistically significant. Consistency levels across studies were obtained and reported in chi-squared (Chi2) and in I2. Based on the Higgins concept (11), a value of I2 above 50% was considered excessively non-homogeneous.
Risk of bias across studies
Funnel plot graphical expressions were chosen to search and identify publication bias.
Additional analyses
As stated by Higgins, categorization of values for I2 as low (i.e., 25%), moderate (i.e., 50%) or high (i.e., 75%) is not appropriate for all circumstances (11); however, homogeneity strengthens the results obtained in any meta-analysis, therefore a cut-off value of 50% was determined suitable in our analysis. A sensitivity analysis and subsequent assay was performed over every outcome analysis considered of high heterogeneity (I2>50%), after exclusions of outliers detected on funnel plot expressions; that assay generated new forest and funnel plots. When no outliers could be found, the hypothesis was that true heterogeneity had occurred.
Results
Study selection
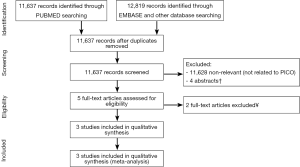
A total of 11,637 records were identified through the MEDLINE search and 12,819 records were identified through EMBASE and other databases searching. After applying inclusion criteria and removing duplicates, nine records were found. Four (12-15) were excluded since they were abstract-only records and had no extractable data. Of the five full-text articles assessed for eligibility, two were excluded. One compared ESD and TEMS and not surgical resection (16), and one did not allow for separate analysis on patients with early-only CRC (17). Three studies were included in the final qualitative analysis and meta-analysis (Figure 1).
Study characteristics
No randomized studies were found within the screened records. All three studies were either prospective or retrospective cohorts, and all three were published in English. Across all three studies, 768 subjects in the experimental intervention (i.e., endoscopic resection) and 552 on the control or comparison group (i.e., surgical resection) were included. Patients with early-only colorectal neoplasia were included in each group. Experimental intervention was either ESD and EMR, or ESD alone. Control intervention was either laparoscopic and open surgery, or laparoscopic surgery alone. The primary outcomes assessed were en bloc resection and curative resection. The Kiriyama study also published 3-year survival data, and was the only one to publish long term follow-up data. The secondary outcomes were procedure duration, hospitalization duration, and complications. Two of the studies also recorded the time needed before dietary intake was restored. Studies characteristics are summarized (see Table 1).

Full table
Risk of bias within studies
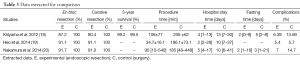
Using the inclusion criteria mentioned above, all three studies scored at least a six on the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies (8). However, when the methods of each study were analyzed, other possible sources of bias were identified, regarding the comparability between the interventional and control groups. In the Kiriyama (18) study and the Nakamura (20) study, whenever deeper, submucosal invasion was suspected on magnified narrow band imaging (NBI) examination, the patient was referred to surgical intervention; when deeper invasion signs were not found, the patient underwent endoscopic resection. Additionally, lesion location and median size in the experimental and control groups varied across studies (Tables 2,3).

Full table
Full table
Results of individual studies
Below we list the characteristics and the available data of the included studies. The number of patients on each group, the age of the patients, the location of the neoplasia (i.e., colonic or rectal), and lesion size were the data available concerning the characteristics of the populations. En bloc resection, curative resection, procedure time, resection time, fasting time, and complications were the outcomes reported on all studies; however, the only comparable outcomes throughout all three studies were en bloc resection, curative resection, and complications. Procedure time was the only continuous outcome variable expressed in mean and standard deviation in two of the selected studies [Kiriyama et al. (18) and Heo et al. (19)]. Survival and follow-up data were only published in one article, Kiriyama et al. (18) (Tables 3,4).

Full table
Synthesis of results
The following figures represent the estimates for risk difference, based on calculated CIs, for each outcome. Risk of bias across studies and additional analysis are also reported.
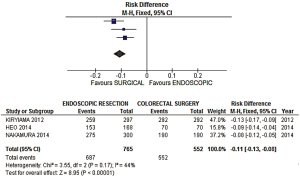
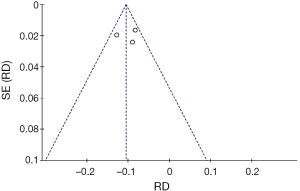
- En bloc resection rate: a risk difference of −0.11 (−0.13, −0.08 95% CI, P<0.00001) was observed, favoring the surgical group, with an acceptable heterogeneity of 44% expressed by the I2 (Figures 2,3);
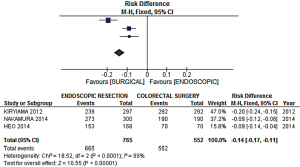
- Curative resection rate: a risk difference of −0.14 (−0.17, −0.11 95% CI, P<0.0001) was observed, favoring the surgical group, with a high heterogeneity level of 89% expressed by the I2 (Figure 4).
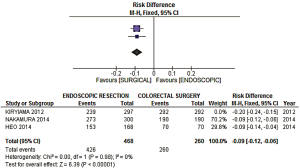
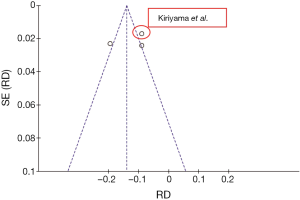
An additional sensitivity analysis was performed after identification and exclusion of one outlier (Figure 5—Kiryiama et al.); a more homogeneous result was then obtained. The adjusted risk difference for curative resection was −0.09 (−0.12, −0.06 95% CI, P<0.00001), with an I2= 0%, favoring the surgical group (Figures 6,7).
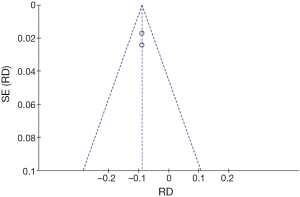
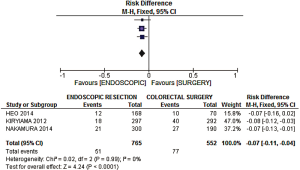
- Complications: a risk difference of −0.07 (−0.11, −0.04 95% CI, P<0.0001) favoring the endoscopic group was observed, with an heterogeneity of 0% expressed by the I2 (Figures 8,9);
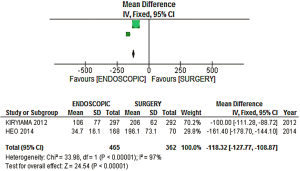
- Procedure time: an analysis of the two studies that reported comparable information on procedure time (i.e., measured in minutes) was performed. A mean difference of −118.32 min (−127.77, −108.87 95% CI) was observed (Figure 10), the longer mean procedure time being found in the surgical group. However, that result was accompanied by true high heterogeneity (I2=97%) across the two studies (Figure 11).
Discussion
Summary of evidence
Interpretations of the above findings are listed below.
(I) En bloc resection: A statistically significant difference was observed in the analysis [risk difference of −0.11 (−0.13, −0.08 CI), P<0.00001], favoring the surgical group, with an acceptable level of heterogeneity across studies. The NNH calculated is 10 (interpretation: after every 10 endoscopic resections of early CRC, one piece-meal resection is performed, when compared with surgical resection).
(II) Curative (R0) resection: After a sensitivity analysis was performed due to high heterogeneity, a statistically significant difference favoring the surgical group was observed [risk difference of −0.09 (−0.12, −0.06 CI), P<0.00001], with a level of heterogeneity across studies equal to 0%. The NNH calculated is 12 (interpretation: after every 12 endoscopic resections of early CRC, one incomplete resection (e.g., R1) is performed, when compared with surgical resection).
(III) Complications: A statistically significant difference, favoring the endoscopic group, was observed in the analysis [risk difference of −0.07 (−0.11, −0.04 CI)], with a level of heterogeneity across studies equal or below 0%. The NNT calculated is 15 (interpretation: after every 15 endoscopic resections of early CRC, one complication is avoided, when compared with surgical resection).
(IV) Procedure time: A statistically significant difference was observed in the complementary analysis for procedure time (mean difference of −118.32 minutes (−127.77, −108.87 95% CI, P<0.00001). However, this information should be carefully analyzed, since the heterogeneity across the two studies included is very high (I2=97%). Using a cut-off target value of 60 minutes, the interpretation of the NNT associated with this mean difference is that for every two endoscopically treated patients, 60 minutes can be spared, as compared with the surgical treatment.
This is the first meta-analysis that summarizes all the data available to compare endoscopic and surgical treatment of early CRC. Although only three studies met our inclusion criteria, the pooled number of patients (768 patients undergoing endoscopic resection and 552 patients undergoing surgical resection) is significant. Considering individual study results, all three studies obtained similar, statistically significant data results concerning en bloc resection, curative resection, and complications, as observed after a careful systematic review. The meta-analysis depicted in this study reinforces some of these findings, and provides solid ground for future developments of larger scale, better designed studies, such as multicenter randomized controlled trials.
Limitations
Our review included three non randomized studies, which could further impair the quality of the data reported. The endoscopic techniques employed for treatment of early CRC are extremely advanced and operator-dependent; and the three studies are from two Asian centers whose good results and great expertise may influence the procedure time results. Differences in the intervention and control populations present as a possible confounding source in all three included studies: lesion location and size differ among the intervention and control groups, and in two of them (Nakamura et al. and Kiriyama et al.), preoperative invasion depth dictated the choice between surgical or endoscopic approaches.
Long-term results are available in one study, thus neither individual analysis nor analytical comparisons are feasible. As explained previously, the choice of treatment in two of the studies (Kiriyama et al. and Nakamura et al.) depended on whether or not more advanced disease was suspected; therefore, long-term results between the intervention and control groups might suffer from this bias of allocation, as more advanced disease results in higher probability of neoplastic dissemination (2,3).
The analysis performed on complications is a difficult one, considering the different morbidity of surgical and endoscopic complications. Nonetheless, endoscopy displayed a lower frequency of complications, which should be understood as an underestimated result—perhaps an analysis of the hospitalization time would be of more use, if there was available data for comparison.
Conclusions
According to the current available data, the treatment of early CRC by surgical resection is associated with higher curative resection rates, higher en bloc resection rates, despite of higher complications rates, as compared to endoscopic resection. Shorter procedure times are associated with the endoscopic methods of treatment, however high heterogeneity levels limit this conclusion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- AJCC (American Joint Committee on Cancer) Cancer Staging Manual, 7th edition. Edge SB, Byrd DR, Compton CC, et al. eds. Springer, New York, 2010:143. ISBN 978-0-387-88440-0.
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130-60. [Crossref] [PubMed]
- Kudo S, Kashida H, Nakajima T, et al. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg 1997;21:694-701. [Crossref] [PubMed]
- Belderbos TD, Leenders M, Moons LM, et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388-402. [Crossref] [PubMed]
- Ikematsu H, Yoda Y, Matsuda T, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 2013;144:551-9; quiz e14.
- Yoda Y, Ikematsu H, Matsuda T, et al. A large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy 2013;45:718-24. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Wells GA, Shea B, O'Connell D, et al. Available online: http://www.ohri.ca/
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Cooper GS, Xu F, Schluchter MD, et al. Endoscopic versus surgical management of Malignant Colon Polyps: A community-based comparative analysis. AGA 2011;140:S-97-S-98.
- Ngamruengphong S, Crowell M, Das A. 683b: Long Term Cancer-Free Survival Is Similar With Either Endoscopic or Surgical Treatment of Malignant Colo- Gastrointestinal Endoscopy Rectal Polyps - Report of an Analysis of the Surveillance, Epidemiology, and End Results Database. Gastrointestinal Endoscopy 2010;71:AB129. [Crossref]
- Tau JA, Cano JJ, Shaib YH, et al. Surgical vs. Endoscopic Management of Colorectal Adenomas With High Grade Dysplasia. AGA 2014;146:S-405.
- Kessels K, Moons LM, Oijen MV, et al. Risk of Colorectal Cancer After Endoscopic vs. Surgical Resection of Carcinoma in Situ Is Not Different. Gastrointestinal Endoscopy 2014;79:AB534-AB535. [Crossref]
- Hon SS, Ng SS, Chiu PW, et al. Endoscopic submucosal dissection versus local excision for early rectal neoplasms: a comparative study. Surg Endosc 2011;25:3923-7. [Crossref] [PubMed]
- Bhangu A, Brown G, Nicholls RJ, et al. Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a Surveillance, Epidemiology, and End Results (SEER) population-based study. Ann Surg 2013;258:563-9; discussion 569-71. [PubMed]
- Kiriyama S, Saito Y, Yamamoto S, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy 2012;44:1024-30. [Crossref] [PubMed]
- Heo J, Jeon SW, Jung MK, et al. Endoscopic resection as the first-line treatment for early colorectal cancer: comparison with surgery. Surg Endosc 2014;28:3435-42. [Crossref] [PubMed]
- Nakamura F, Saito Y, Sakamoto T, et al. Potential perioperative advantage of colorectal endoscopic submucosal dissection versus laparoscopy-assisted colectomy. Surg Endosc 2015;29:596-606. [Crossref] [PubMed]