Perioperative outcomes associated with robotic Ivor Lewis esophagectomy in patient’s undergoing neoadjuvant chemoradiotherapy
Introduction
In 2013 there were will be an estimated 17,990 new cases of esophageal cancer and 15,210 deaths from the disease in the United States (1). The prognosis for patients with locally advanced esophageal cancer (defined as ≥ T2 or node positive disease) is poor, with five-year survival rates ranging from 15-34% (2,3). Surgery alone is associated with poor long-term outcomes and definitive chemoradiation has a high locoregional recurrence rate in locally advanced esophageal cancer (4-6).
There has been conflicting evidence about the utility of neoadjuvant chemotherapy in the management of patients with locally advanced esophageal cancer (7-16). Some of these studies were limited by inadequate pre-operative staging; as well as by heterogeneity of patient population including both adenocarcinomas and squamous cell carcinomas (SCC). In spite of the conflicting evidence from some of the earlier trials, neoadjuvant chemoradiation (NCR) is currently considered the standard of care in patients with locally advanced esophageal cancer. It is currently included in the National Comprehensive Cancer Network Guidelines (NCCN) (17). In general, patients who have a response to neoadjuvant chemotherapy have improved disease free survival (DFS) and overall survival (OS) compared to patients that do not (18).
Ivor Lewis esophagectomy (ILE) is a common surgical approach for esophageal resection. The other approaches that are routinely used are the transhiatal esophagectomy (THE) and three-field esophagectomy (TFE). ILE is performed using both a right anterolateral thoracotomy incision and an abdominal incision. A significant source of the morbidity from this approach is due to the right thoracotomy. These complications include significant post-operative pain, atelectasis, pneumonia and atrial fibrillation (Afib) with pulmonary and wound complications being the most common morbidities associated with the transthoracic approach.
Minimally invasive esophageal surgery (MIE) has been increasingly used in patients undergoing surgery for esophageal cancer (19-21). Potential advantages of MIE include the decreased post-operative pain; lower post-operative wound infection, decreased pulmonary complications, and decreased length of hospitalization (LOH). Robotic ILE is a new technique in the armamentarium for MIE surgery (22,23). Robotic esophageal surgery has the ability to overcome some of the limitations of laparoscopic and thoracoscopic approaches to esophagectomy. Specifically, it allows for a broader view of the operative field in the mediastinum, three-dimensional camera views, as well as greater range of instrument motion and articulation.
There is limited data on the impact of NCR in patients treated with robotic-assisted ILE (RAIL). We sought to examine our single institution experience with this technique in patients undergoing neoadjuvant chemo-radiation and to investigate primary operative and oncologic outcomes. To date this represents the largest series of consecutive patients treated with robotic Ivor-Lewis esophagectomy with or without NCR.
Methods
A query was performed from an Institutional Review Board (IRB) approved, prospectively maintained database of patients undergoing RAIL between October 2010 and June 2012. Perioperative morbidity and mortality were compared in the cohort of patients who received NCR vs. the cohort that did not receive NCR.
Endpoints and statistical analysis
The primary operative endpoints were median operating room (OR) time, estimated blood loss (EBL), intensive care unit (ICU) days following surgery, and LOH. Secondary end-points included peri-operative adverse events (AEs) less than 30 days following surgery; including pneumonia, Afib, deep vein thrombosis (DVT)/pulmonary embolism (PE), wound infection, leak, and death.
Statistical analysis was performed using SPSS® version 21.0 (IBM®, Chicago, IL, USA). Continuous variables were compared using the Kruskal Wallis or the ANOVA tests as appropriate. Pearson’s Chi-square test was used to compare categorical variables. All statistical tests were two-sided and an α (type I) error <0.05 was considered statistically significant.
Neoadjuvant chemoradiation therapy
Patients were discussed in a weekly multi-disciplinary tumor board conference and pathology was reviewed at our institution. Pre-operative staging including endoscopic ultrasound, CT chest, abdomen and pelvis and PET scan as per NCCN guidelines (17). Patients who had locally advanced disease (≥ T2 and/or ≥ N1) were treated with neoadjuvant chemoradiation. The choice of chemotherapy was left to the discretion of the treatment medical oncologist.
Patients treated at our institution receive infusional 5-fluorouracil (5-FU) and cisplatin, with concurrent external beam radiation for a total dose of 50.4 Gy over the course of 5-6 weeks. Six weeks after the conclusion of therapy, patients restaging PET-CT scans were performed. Patients without evidence of metastatic disease and good performance status were then offered esophagectomy. Esophagectomy was performed during the 6-12 week window after conclusion of chemoradiation. Patients are referred to cardiac and pulmonary specialists for pre-operative risk assessment and optimization prior to surgery.
Surgical technique
Our surgical technique for RAIL has been previously described (22,23) using the DaVinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). Of note, no patients underwent pyloric emptying procedure. Rather, in lieu of a pyloromyotomy, three-hundred units of botulinium toxin (Botox®) were injected into the pylorus. A feeding jejunostomy tube was placed routinely in all the patients.
The thoracic portion of the procedure was done entirely with the robot using three incisions. Mediastinal lymph node dissection of levels 7, 8, and 9 was routinely performed. Intra-operative frozen section was used to confirm negative margins. The anastomosis was performed either via a circular stapling technique, or handsewn.
Post-operative care
Patients were extubated in the operating or recovery room and admitted to the ICU overnight for observation. On post-operative day 4 or 5, an esophagram is obtained to identify leaks and delayed gastric emptying. If the esophagram is normal, the nasogastric tube was removed and clear liquid diet started. The chest tube was removed once patients were tolerating a regular diet and there was no evidence of chyle leak.
Results
Patient demographics
Eighty-nine patients underwent RAIL during the study period. Seventy-seven patients (87%) had NCR and 22 patients did not (13%). The median age was 66 (range, 44-83), there were 69 men (80%). The median age of the patients treated with NCR was younger than the patients in the non NCR group {69 [44-83] vs. 64 [46-81] years respectively, P=0.05}. The patients who underwent NCR had a higher BMI then those who went straight to esophagectomy. The median overall BMI was 28 kg/m2. The mean BMI for those receiving NCR was 31±5 and 27±5 for those who did not (P=0.001). Seventy-seven patients had adenocarcinomas (86%), 8 (9%) patients had SCC, 3 (3%) patients had neuroendocrine tumors and two patients (2%) had high-grade dysplasia (HGD). Patients treated with NCR had a higher pre-operative BMI compared with patients treated with RAIL alone (P=0.001) (Table 1).
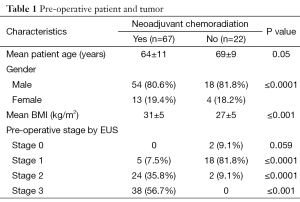
Full table
Perioperative outcome
There were no conversions to open laparotomy or thoracotomy in either group. There were no statistically significant differences in the mean operative times and EBL between both groups. The mean operative time in the group treated with NCR was 434±89 and 427±82 minutes in the cohort of treated with surgery alone (P=0.74). The mean EBL was 149±98 mL in the NCR and 153±78 mL in the group treated with surgery alone (P=0.52).
Post-operative complications
Complications occurred in 17 (19.1%) patients. There were no statistically significant differences in the rates of any complications between patients receiving NCR n=10 (15%) and those that did not receive NCR n=7 (32%) (P=0.11). The post-operative complications included Afib 6 (6.7%), pneumonia 7 (7.9%), anastomotic leak n=2 (2.2%), conduit staple line leak n=1 (1.1%) and chyle leak n=1 (1.1%). A-fib occurred in 4 (6%) of the NCR patients and 2 (9%) of the non NCR patients (P=0.63). Of the 7 patients who developed pneumonias, 4 (6%) were in the NCR cohort and 3 (13.6%) in the non NCR group (P=0.36). The two anastomotic leaks occurred in the patients in the patients treated without NCR and one gastric conduit leak in the patient treated with NCR (P=0.12). There were no deaths in either group (Table 2).
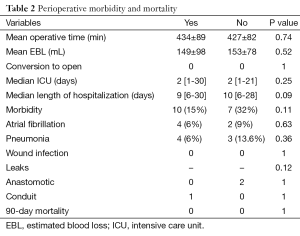
Full table
The total number of days in hospital and total number of ICU days were also similar in both groups (Table 2). The NCR patients spent a median of 9 [6-30] days in the hospital compared to the non NCR group who spent a median of 10 [6-28] days hospitalized (P=0.09). The median ICU stay for both groups was 2 [2-30] days (P=0.25). Those patients who developed a complication had a median LOH of 15 days [7-30] compared to 9 [6-21] days in those who did not experience a complication (P<0.0001).
Pathology
The tumor was located in the mid esophagus in 7 (7.8%), and in the lower third or gastro-esophageal junction in 82 (92.2%) of patients. All patients underwent an R0 resection. Of the 67 patients receiving NCR, there were 22 (32.8%) who exhibited a pathological complete response (pCR), 32 (47.8%) with a partial response, and 13 (19.4%) who exhibited no response. There was no statistically significant difference in the mean number of lymph nodes harvested in the patients treated with NCR compared with those treated without NCR. The mean number of LN’s harvested in the NCR group was 20.2±8.4 compared to 21.7±11 in the non NCR cohort (P=0.5) (Table 3).

Full table
Discussion
We report our series of 89 patients who underwent RILE with or without receiving neoadjuvant therapy. We demonstrated that there was no increase in operative time or EBL in patients receiving NCR compared to those who did not. Additionally, incidences of post-operative complications including anastomotic leak, Afib, wound infection, chylous thorax, and pneumonia did not differ between cohorts. No mortalities were noted in either group. Length of ICU stay and hospitalization was similar between groups and not found to be statistically significant. As to be expected, patients in either cohort who developed a complication exhibited an increase in LOH.
Esophageal cancer was the seventh leading cause of death in the United States in 2012 (24). For tumors invading beyond the muscularis propria or involving locoregional lymph nodes, surgery alone is associated with dismal survival thereby necessitating a multimodality approach employing chemoradiation (3,4,7,16). The response to neoadjuvant chemoradiation continues to be one of the most important factors in predicting OS in patients undergoing NCR (18,25). The CROSS trial randomized patients with the resectable esophageal cancer to receive surgery alone or chemotherapy followed by surgery. The median OS was 49.4 months in the chemoradiotherapy-surgery group vs. 24 months in the surgery group (HR, 0.657; 95% CI, 0.495-0.871; P=0.003) (16). In patients who underwent NCR, the pCR rate was 29%.
Esophagectomy alone remains the cornerstone treatment for early-stage esophageal carcinoma. Compared with surgery for other gastrointestinal malignancies, esophagectomy has higher morbidity and mortality rates. Data does suggest that performing esophagectomy in a high volume reduces the post-operative complication rate and mortality (26). Pulmonary and wound complications contribute to the majority of the morbidity following a transthoracic esophagectomy. The minimally invasive approach to esophagectomy offers the potential to reduce pulmonary complications, wound infections, post-operative pain, improve recovery times, and shorter lengths of hospitalization (27).
The initial trials of MIE involved the mobilization of the gastric conduit laparoscopically with completion of the thoracic portion of the procedure done either thoracoscopically or through a small thoracotomy (22); however this approach still has the potential for increased wound and pulmonary complications. A retrospective review of 530 patients treated with minimally invasive total minimally invasive ILE treated at the University of Pittsburgh, demonstrated an operative mortality rate of 1% with a median length of stay of 7 days (28).
A multicenter, open-label, controlled trial of 115 patients randomized to open or minimally invasive transthoracic esophagectomy, demonstrated significantly lower rates of pulmonary infections in patients undergoing MIE (24% vs. 12%, P=0.005). The length of stay was also shorter in those patients undergoing less invasive techniques (11 vs. 14 days, P=0.044) (27). Retrospective data and case series have also demonstrated that minimally invasive approaches are safe and have comparable oncologic outcomes (29).
Robotic esophageal surgery has the ability to overcome some of the limitations of laparoscopic and thoracoscopic approaches to esophagectomy. Specifically, it allows for a broader view of the operative field in the mediastinum and greater range of instrument motion. The data regarding the safety and oncologic outcomes in patients undergoing robotic-assisted esophagogastrectomies is limited, with fewer than a hundred patients published in the surgical literature.
The utility of the robotic esophagectomy in patients undergoing treatment with NCR is unknown however it may allow improved visualization of the operative field and more precise dissection and manipulation of friable tissues. We have demonstrated that in patients who were treated with NCR and underwent robotic approaches to esophageal resection, there was no increase in AEs compared to those who did not receive NCR. Additionally, there were no differences in adverse outcomes when comparing to historical minimally invasive approaches (data not shown).
Due to its retrospective nature, there are potential for selection bias. We currently offer the robotic approach to all patients regardless of their treatment characteristics, age, or BMI. Additionally patients must be able to tolerate the operation from a cardiopulmonary standpoint. There were no differences in age amongst groups and the NCR group actually had higher BMI’s then the non NCR cohort. Also we report all consecutive patients undergoing the approach thereby limiting patient selection bias in our data.
Conclusions
We have demonstrated that RILE is a safe and feasible option for patients with esophageal cancer. The administration of neoadjuvant chemotherapy to RAIL did not result in an increase in perioperative morbidity and mortality. Operative time, and EBL was not impacted by patients undergoing NCR. The number of lymph nodes harvested and the completeness of resection was also similar between patients who received neoadjuvant chemoradiation and those who did not. Longer follow-up is required in order to determine long term oncologic outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [PubMed]
- Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2003;185:538-43. [PubMed]
- Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306-13. [PubMed]
- Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. [PubMed]
- Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 1999;230:392-400; discussion 400-3. [PubMed]
- Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg 1992;16:1104-9; discussion 1110. [PubMed]
- Lv J, Cao XF, Zhu B, et al. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 2010;16:1649-54. [PubMed]
- Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 1994;73:1779-84. [PubMed]
- Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology 1994;41:391-3. [PubMed]
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [PubMed]
- Lee JL, Park SI, Kim SB, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol 2004;15:947-54. [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers. Version 2, 2013. 2013. Accessed June 21, 2013. Available online: www.nccn.org
- Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159-67. [PubMed]
- Galvani CA, Gorodner MV, Moser F, et al. Robotically assisted laparoscopic transhiatal esophagectomy. Surg Endosc 2008;22:188-95. [PubMed]
- Dunn DH, Johnson EM, Morphew JA, et al. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus 2013;26:159-66. [PubMed]
- Kernstine KH, DeArmond DT, Shamoun DM, et al. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc 2007;21:2285-92. [PubMed]
- Yamamoto M, Weber JM, Karl RC, et al. Minimally invasive surgery for esophageal cancer: review of the literature and institutional experience. Cancer Control 2013;20:130-7. [PubMed]
- Hernandez JM, Dimou F, Weber J, et al. Defining the learning curve for robotic-assisted esophagogastrectomy. J Gastrointest Surg 2013;17:1346-51. [PubMed]
- Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212-36. [PubMed]
- Dittrick GW, Weber JM, Shridhar R, et al. Pathologic nonresponders after neoadjuvant chemoradiation for esophageal cancer demonstrate no survival benefit compared with patients treated with primary esophagectomy. Ann Surg Oncol 2012;19:1678-84. [PubMed]
- Hollenbeck BK, Dunn RL, Miller DC, et al. Volume-based referral for cancer surgery: informing the debate. J Clin Oncol 2007;25:91-6. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes After Minimally Invasive Esophagectomy: Review of Over 1000 Patients. Ann Surg 2012;256:95-103. [PubMed]
- Santillan AA, Farma JM, Meredith KL, et al. Minimally Invasive Surgery for Esophageal Cancer. J Natl Compr Canc Netw 2008;6:879-84. [PubMed]


