Soft tissue tumors of the anorectum: rare, complex and misunderstood
Introduction
Anorectal soft tissue tumors (ARST) are a heterogeneous group of uncommon neoplasms that range from aggressive malignancies to benign but symptomatic lesions located in an anatomically complex site. ARSTs account for approximately 1% of all small and large bowel tumors (1), and constitute less than 0.1% of colon and rectal malignancies (2). Since the first case report in 1881 (3), a number of isolated case reports and small, single institution series have been published. The majority of cases described are gastrointestinal stromal tumors, and only a few papers address the rarer ARSTs. Due to the rarity of these cases, there is an obvious lack of randomized trials and well-defined practice guidelines for treating these patients. However, with the ever-increasing use of abdominopelvic imaging (computed tomography and magnetic resonance), it might be expected that an increasing number of ARSTs will be diagnosed. As such, we set out to review the current understanding of diagnosis and treatment of these lesions.
Methods
A literature search was conducted using Pubmed and Embase electronic databases. The following MESH terms were used for disease location: “rectum” “anus”, “perianal”. “Colon” and “sigmoid” were included in our search, to avoid missing anorectal cases reported in broad under “colon” or “sigmoid” series. In addition to “soft tissue neoplasms”, MESH terms of the following more frequent types of soft tissue disease were used: “leiomyosarcoma”, “angiosarcoma”, “liposarcoma”, “dermatofibrosarcoma protuberans”, “malignant fibrous histiocytoma”, “rhabdomyosarcoma”, “neurilemmoma”, “solitary fibrous tumor”, “gastrointestinal stromal tumor” and “desmoid tumor”.
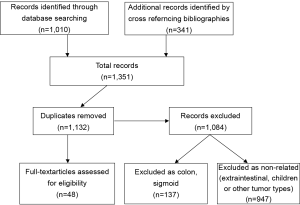
All articles related to humans and published in English between 1980 and 2011 in peer-reviewed journals were considered. The articles retrieved were reviewed independently by two of the authors (MON and ANM). To ensure that all relevant publications were captured, we performed a second literature search by cross-referencing bibliographies of all previously retained articles. Duplicate articles as well as those without a specific anorectal focus were then discarded. A total of 48 articles were retained from an initial list of 1,351 publications (Figure 1), based on abstract review. These 48 papers then underwent complete manuscript review and data extraction to be included in this report (Table 1).
Full table
Findings
Leiomyosarcoma
Leiomyosarcomas (LMS) are malignant soft tissue neoplasms arising from smooth muscle tissue located within the muscularis mucosa, muscularis propria and blood or lymphatic vessels (4). LMSs are rare, but are the most common histological type of ARST, making up over 90% of reported cases (5). In a 2000 review by Hatch et al., 480 anorectal LMS cases were identified in the literature (6). They found the peak incidence of cases occurred at 50-69 years of age and only 6.4% of them were located to the anus. There seems to be a male predominance for LMSs of the rectal region and a female predominance for tumors occurring within the anal canal (7). Anal lesions are often plaque-like protrusions arising intra-murally or sub-mucosally from the posterior aspect of the anorectum, with areas of pressure ulceration (8-10).
Histologically, LMSs feature spindle cells with elongated, blunt-ended nuclei in an eosinophillic cytoplasm. These cells exhibit a fascicular growth pattern originating from vascular tissue or muscularis mucosa (11). LMS frequently exhibit high mitotic activity, often greater than 50 mitosis per 50 high-powered fields (HPF) (12). Immunohistochemically, these tumors are positive for vimentin, actin, smooth muscle myosin and desmin, but are CD34, CD117 and K-RAS negative (12-15). Because of histological similarities, LMSs are often misdiagnosed as leiomyomas. K-RAS negativity, high mitotic activity, large tumor size, nuclear and cellular atypia as well as large size of the tumor and the presence of extensive necrosis are useful in confirming the diagnosis of LMS (16).
Almost all the cases of anorectal LMS reported in the literature were treated with surgical excision (abdominoperineal resection (APR) or wide local excision). Operative choice was most often dictated by high cellularity and tumor size rather than anatomic location (6). Higher rates of recurrence have been reported with local excision (67%) compared to APR (19%) in some series (17). In contrast, other series report similar outcomes regardless of surgical approach (18,19). Anorectal LMSs were traditionally thought of as resistant to both chemotherapy and radiation. However, this notion is largely based on sporadic case reports (19) or publications predating the advent of megavolt radiotherapy. More recent experience suggests a possible role for pre-operative chemotherapy and/or radiotherapy in sphincter preserving surgeries, but the relatively small series do not allow definitive conclusions (10,20,21).
LMSs rarely metastasize through lymphatics and are more likely to spread through the lungs and liver through hematogenous spread (22-24). Recurrence rates for anorectal LMS are associated with high histological grade, large tumor size and incomplete surgical resection (16). Five year survival reported as varying between 20-25% with poor prognosis overall in rectal leiomyosarcomas (25-28).
Rhabdomyosarcoma
Rhabdomyosarcoma (RMS) is one of the most common childhood soft tissue tumors, but represents less than 5% of malignant soft tissue lesions in adults (29-31). Anorectal presentation is extremely rare and is seen in less than 2% of cases (32). In this location, these tumors are thought to arise from the muscular layers of the bowel and the genitourinary tract. Most of the data on this disease originates from studies conducted between 1972 and 1997 by the Intergroup RMS Society for the RMS population aged 0-21 years (33). In these studies, cases with anorectal involvement were analyzed separately and a practice guideline has been developed (discussed below) (34). Although, designed originally for children, it is also used for adults, due to the small number of RMS cases in this population (35). It has been postulated that perineal RMS occurring at a younger age may have a genetic predisposition and is related to other genetic syndromes such as Nijmegen breakage syndrome (36). No similar syndromic associations have been observed in adults.
RMS is described as a grossly uncircumscribed lesion with multiple areas of spherical growth, often resembling a “bunch of grapes” that is soft in consistency. Histologically, it is divided into three major types: embryonal, including spindle and botryoid subtypes, alveolar and undifferentiated (37). As a result of its mesodermal/muscle tissue origin, RMSs tend to show multiple areas of muscle tissue at different stages of development. Embryonal type shows a spindle shape pattern often arranged in patches of highly cellular areas alternating with sparsely cellular areas containing mucoid cytoplasm which is often a hallmark of the diagnosis (38). Immunohistochemically, these tumors react positively to a number of stains, including myogenin, myoD1, sarcomeric actin, myosin, desmin and vimentin; however alpha actinin, z-protein and titin are more specific to the condition (39-41). The alveolar subtype has a gross appearance of more circumscribed edges and a firmer, more rounded consistency. Microscopically, these tend to contain rounded, small acidophilic cells septated by collagen tissue with a broken off appearance with occasional giant cell formation. They tend to have larger nuclei and heavier chromatin patterns than embryonal types. Immunohistochemical profiling is the same as embryonal but staining for anti-skeletal muscle antibody and S100 protein was also documented (42,43). A study of 24 RMS of different types, showed all cases staining positively for Ankyrin-Repeated Proteins “Arpp/Carp” (44).
RMS is harder to diagnose in adults, with more advanced disease at presentation (34). This may be due to the fact that symptoms (tenesmus, pain on defecation or fever along with mild leukocytosis in some cases) tend to present later, probably because of a larger space for the tumor to grow. There is also a higher risk of misdiagnosis with diseases of infectious or inflammatory origin, such as perianal abscesses (45-47). In addition, younger patients and especially infants tend to be examined more often by parents during diaper changes possibly explaining why RMS is picked up earlier (34).
The mainstay of RMS treatment is resection to negative margins and a combination of chemotherapy and radiation therapy. Pediatric RMS guidelines state that microscopically negative margins should be obtained and ipsilateral lymph node basins (inguinal, retroperitoneal) should be excised in patients older than 10 years. Although unproven, these guidelines have been used for treating adults as well. The use of chemotherapy combining Vincristine, Actinomycin D, Cyclophosphamide and Adriamycin in conjunction with external beam radiotherapy for patients with positive margins, and local node involvement has also been advocated (34). However, radiotherapy is often associated with dermopathy, anal stenosis and genitourinary complications (48). Because aggressive surgery often results in sacrificing the function of the anorectum, sporadic attempts have been made at neoadjuvant chemotherapy to reduce tumor size and improve resectability. Intraoperative radiation therapy also has been sporadically used.
Twelve adult cases of anorectal RMS have been reported (45-47,49-56), ranging between the ages of 18 and 71. Seven patients had radical surgery (APR or anterior resection), three had local excision and two never underwent an operation (patient refusal and bony metastasis at presentation. Half the patients had adjuvant chemoradiation with a combination of Vincristine, Actinomycin-D, Adriamycin and/or ifosfamide. Neoadjuvant therapy was used in two patients. Ten of the twelve cases had follow up data reported. Among these, 3 patients recurred within 6 months of surgery. There were 5 deaths, including the 2 patients that never underwent operation. The longest disease-free follow up period was 12 years (54). Interestingly, the patient having undergone transanal excision was reported to be disease free 7 years post resection (55).
More recently, these efforts appear to have reduced perioperative morbidity and mortality (35,57). Watanabe et al. reported that patients given neoadjuvant chemotherapy followed by local excision had better tumor free survival and lower local recurrence rates than that of those treated by APR without neoadjuvant chemotherapy in addition to the anal function preservation. Nevertheless, we had decided to exclude this series because it was based on personal communications and cases reported in the Japanese language literature.
It is thought that because of the delays in diagnosis discussed above, the prognosis of RMS in adults is worse than the younger age groups. The 5-year disease free survival (DFS) is 18% in adults versus 65% in children and the overall survival (OS) of 20% in Adults versus 71% in children (34). Other factors influencing survival include regional lymph node involvement (5 year DFS of patients without lymph node involvement was 63% versus 32% for node positive cases), evidence of distant metastasis and primary tumor size (5 year DFS of 71% in tumors less than 5 cm compared to 31% in those greater).
Angiosarcoma
Angiosarcoma (AS) accounts for 4.1% of all soft tissue malignancies. This disease has undergone numerous name changes (hemangioendothelioma, hemangiosarcoma, malignant angioendothelioma and lymphangiosarcoma), reflecting progressive growth in our understanding of tumors originating from vascular and lymphatic walls (58). AS is more frequent in Caucasians and males (59), is associated with radiation exposure (60) and has been linked with chemotoxins such as vinyl chloride, arsenic, thorium dioxide as well as long-term exposure to drugs such as androgens (61,62). Four of the anorectal AS cases we identified (25%) had prior history of pelvic irradiation supporting the association of radiation with AS incidence (63-66). One patient had a longstanding foreign body in the pelvis from previous surgery (67) while another had previous history of chronic rectal ulceration from recurrent abscess and fistulae. Both these cases support the suggestion that some sarcomas originate from sites with chronic inflammation (68).
ASs present as firm, highly vascular lesions that may be mistaken for carcinoma or melanoma on gross pathological examination. Microscopically, hematoxylin and eosin (H & E) staining alone can be difficult in yielding a positive diagnosis of malignancy as it will show vascular channels lined with only subtly abnormal endothelial cells, often mimicking benign hemangiomas (69). Because of this relative lack of specific morphology, and the frequent absence of the diagnostic abnormal vascular channels in the poorly differentiated forms, immunohistochemical stains are often employed to confirm the diagnosis and they include vimentin, CD 31, CD 34 and BNH9 (an endothelial marker). Epithelial markers such as CAM 5.2 are used to confirm the presence of an epithelioid variant of AS (70-72).
There are no clear guidelines on the management of anorectal AS. We know from other sites that surgery and radiation therapy have an important role. For example, in a retrospective review of 67 patients with non-anorectal AS, Mark et al. showed a 5-year disease-free survival of 43% in patients who underwent surgery and radiation as opposed to 17% in patients who underwent surgery without radiation (73). The role for chemotherapy on the other hand is still under investigation, with some response reported with Paclitaxel, Docetaxel, Doxorubicin and Daunorubicin (74).
There are 12 cases of AS of the rectum reported in the literature and none of the anus (63-68,75-79). Among these, one had metastasis to bone and two had lymph node involvement at the time of diagnosis (66,67,76). Average age at presentation was 57 years (range, 30-79) and 75% of patients were women. Tumor size ranged between 2 and 16.5 cm (average: 5 cm).
Eight patients underwent surgical excision: 6 radical resections (APR or anterior resection) and 2 local excisions. Of these, 6 also received adjuvant radiation therapy. Of the four non-surgical cases published, 2 were treated with radiotherapy and no treatment details were provided for the remaining 2. Seven of these publications reported follow up data. The longest disease-free survival was 27 months in a young patient treated by posterior exenteration followed by chemotherapy and radiation. Three patients were reported to have died of their disease, all within 8 months. Raising questions about the appropriateness of their preoperative staging (66,67,79).
There are too few anorectal AS cases to support prognostic associations, however, a recent review of colon AS has shown that tumor size (>5 cm), node positivity and distant metastasis all correlated with poor prognosis (80). At the moment, two phase II trials are studying the use of bevacizumab with radiation in the treatment of AS (74). Although these trials do not specifically target anorectal AS, it is hoped that positive findings would translate into easier treatment planning for AS of the anus and rectum.
Dermatofibrosarcoma protruberans
Dermatofibrosarcoma protruberans (DFSP) is thought to arise as a result of the chromosomal translocation t[17;22] in 90% of cases. As a result, the COL1A1 gene fuses with a platelet derived growth factor (PDGF) gene in fibroblasts, leading to over production of PDGF, which is a growth stimulant, thinking it is a structural protein. Fibroblasts contain the receptor for PDGF and thus further stimulating release, growth and mitosis (81,82). DFSP has a 0.4% incidence of distant metastasis, but close to 25% local recurrence rate (83,84). This is attributed to the tentacle-like projections of the tumor into the subcutaneous tissue, making the assurance of adequate negative margins one of the key prognostic factors in the management of DFSP (85).
The incidence of DFSP is thought to be 4.2 per million people annually in North America, with the vast majority of cases (42%) occurring in the truncal area and the remainder in the upper extremity, lower extremity, head and neck (83).
DFSP appears as a solid, nodular polypoid tumor almost always arising from the dermis, with invasion into the subcutaneous tissue. Microscopically, it is characterized by a whorl-like spindle cell pattern of monomorphic fibroblast growth, accompanied usually by a low mitotic activity. In cases where the subcutaneous layer is invaded there is often evidence of entrapment of adipose tissue between extending “limbs” of fibroblast growth. Immunohistochemically, DFSP lesions often exhibit vimentin and CD34 reactivity with occasional focal actin staining and are often negative for factor XIIIa, keratin and S-100 proteins (86).
The treatment of DFSP is surgical and the standard is wide local excision with at least 2 cm margins (87). Given the high recurrence rate, a negative margin is of outmost importance but is hard to achieve in areas close to critical perineal structures such as the anal sphincters and therefore MMS should be considered, especially for small, distal and superficial lesions (88). There have been numerous analyses comparing wide excision to MMS for other disease sites, most with small patient numbers and varying outcomes. A retrospective review comparing the two in 48 patients concluded that although MMS provided fewer incidences of positive margins, it also required longer operative time, higher cost and a higher incidence of complex closure requiring graft or flap (89).
Given that 90% of DFSP cases have a t[17;22] chromosomal translocation and over expression of the (PDGF) gene explained previously, clinical trials with imatinib mesylate have recently been conducted. In a pool of 24 cases from two phase II trials in which daily imatinib was given to patients with locally advanced or metastatic DFSP, 45.9% of the patients showed at least partial response (90). These trials and other reports set the ground for the use of imatinib in the neoadjuvant setting and a phase II trial of 21 patients with positive fusion gene “COL1A1 & PDGF” (91). Eight patients (31%) had complete or partial response with a median 20% decrease in size. This limited data opens the possibility of considering imatinib mesylate preoperatively for anorectal DFSP, in cases where the extent of excision is an issue.
There are only two reported cases of DFSP involving the anorectal region (92,93). One was a four year old child with no co-morbidities and a background history of trauma to the region. The tumor initially involved the right scrotum and then extended onto the anal margin. The lesion was excised using Mohs micrographic surgery (MMS) (92). The second was a 67 year old man who presented with a 3-cm irregular, mobile, elevated, red, polypoid lump at the edge of the anus. MRI of the pelvis showed the mass extending into the ischiorectal space with no sphincter involvement. The patient underwent wide excision, however margins were involved and re-excision with wider margins using skin rotation flaps was required. In both cases sphincter preservation and long term follow up were not reported (93).
Malignant fibrous histiocytoma
Malignant fibrous histiocytoma (MFH) was first described in 1963 by Ozello and Stout (94,95). Its existence as a distinct entity started being questioned in 1987 by Fletcher (96,97). By 2008 it was generally agreed that MFH was not a distinct disease (98). In fact It has been shown with immunohistochemistry that 63% of these tumors are other histological types “mainly liposarcomas” and the remainder were classified as myxofibrosarcoma, angiomatoid fibrous histiocytoma and undifferentiated pleomorphic sarcoma (99). Nevertheless prior to this change in disease classification, eight cases of anorectal MFH were reported (100-107). When confronted with this pathological diagnosis the appropriate course of action should be to request further immunohistochemistry for more accurate diagnosis.
Solitary fibrous tumors
Various terms have been used to describe solitary fibrous tumors (SFT), since their initial description in 1931 (108), including localized fibrous mesothelioma, fibrous tumor of the pleura, fibromyxoma and submesothelioma (109). SFT has been described in many non-pleural sites (110-114). Little is known about the natural history and malignant potential of these; however the literature suggests that the majority (78-88%) are histologically benign (115).
Morphologically, SFTs are well-circumscribed, non-encapsulated, yellow or grey-white lesions, with a firm consistency. They rarely show cystic degeneration or necrosis upon imaging (116). Microscopically, SFTs form spindle cells which may be arranged in a storiform pattern or haphazardly along with fibroblast-like cells arranged in a “patternless pattern”. There are variations in cellularity and cystoplasmic volume. The fibroblast-like cells and spindle cells appear between collagen fibers in a keloid-like formation with blood vessels arranged in a hemangiopericytoma-like pattern. Lipomatous and lymphatic tissue may also be present within the tumor sections (117-120). The presence of necrosis, hemorrhage, increased atypia and high mitotic rate (greater than 1 per 10 HPF) are considered signs of malignant potential (120). On immunohistochemical staining, SFTs are CD 34, vimentin and Bcl-2 positive and negative for keratin and S100 (121,122). They occasionally exhibit desmin positivity, suggesting that combined CD 34 and Bcl-2 staining would yield a definitive diagnosis in borderline cases where similarity to other tumors such as fibrosarcomas and giant cell angiofibromas makes a definitive diagnosis problematic (122).
The principles of management of SFTs are based on how pleural SFTs are treated. Surgical excision with pathological negative margins is the norm. The addition of adjuvant radiotherapy has been reported in select cases, when there is incomplete resection of the tumor especially for the malignant variety. Although ifosafamide and doxorubicin have been reported beneficial for recurrent or inoperable SFT, repeat surgical resection should be sought first. Also, neoadjuvant radiation therapy or brachytherapy have been described for large malignant tumors although this is not supported by evidence (123).
Generally SFTs carry a good prognosis, with low recurrence and metastasis rates. In fact, systemic spread is described in only 8% of cases reported in the literature (115). Higher rates of local recurrence are generally reported for extra-pleural SFTs probably due to smaller excision margins in relation to the anatomic localization of the tumor (118). Positive margins, tumors size greater than 10 cm or malignant histology, are risk factors for local failure for extra-pleural SFTs (118). One study found that the rate of local recurrence is 8 fold greater in cases with malignant features (115). The metastatic potential of extra-pleural SFTs appears to be low (124).
There are only 5 reported cases of anorectal SFT (124-128). Two cases occurred within the ischioanal fossa, one was of rectal origin and reoccurred in the perineum, one originated in the mesorectum and one involved both the rectum and uterus. Four out of 5 patients were male and mean age at diagnosis was 45. Average tumor size was 10 cm (range, 7-13 cm). All cases were treated surgically. Two patients had excision of the tumor through an abdominal approach, with sparing of the rectum (126,127); two patients underwent an APR (124,128), one underwent a perineal extra-peritoneal procedure. None of the patients received adjuvant chemotherapy or radiation.
Follow up data was available in only 3 out of the 5 cases, and ranged from 6 months to 13 years. Both patients who underwent APR had local recurrence, at 6 months (124) and 13 years (128) and received radiotherapy with embolization of the internal pudendal arteries to reduce the tumors size prior to re-excision. No systemic metastasis has been reported from any of the anorectal SFT cases.
Excision with clear margins should always be the goal for anorectal SFT, however, optimal margin size remains unknown. Radiotherapy should be reserved for cases with malignant features, positive margins, unresectablility or preoperatively in the case of recurrence (115). However, radiation does not replace proper negative margin surgical technique. Similar to SFT of the pleura, systemic therapy with ifosfamide or doxorubicin may be considered in recurrent cases or those that show malignant features (123).
Schwannoma
While historically viewed as a subtype of gastrointestinal autonomic nerve tumors (GANTs), schwannomas are now regarded as a separate entity due to their unique immunohistochemical characteristics (129-131). These tumors are thought to be benign with little risk of malignant transformation (132). They arise from neural crest cells and can therefore occur in any anatomical region (133). Von Reckelhausen’s disease, familial adenomatous polyposis and multiple endocrine neoplasia type 2b have all been reported as risk factors for the condition (134).
Grossly these tumors often appear as firm yellow or brown colored lesions which may be pedunculated or sessile. They are almost always restricted to the sub-mucosa, extending from the lamina propria with an aggregate band or surrounding coating of white lymphoid tissue and can vary in size between 1.5-12 cm owing to their largely benign slow growing nature (135). Histologically, they can be divided into 3 subclasses plexiform, epithelioid and spindle cell. Spindle cell schwannomas are characterized by spindle cells arranged in a trabecular pattern with septations by fibrovascular tissue, no verocay bodies and weak nuclear palissading and are often encapsulated by a discontinuous cuff of lymphoid hyperplasia. Mitotic activity rarely exceeds 5 per 50 HPF and nuclear atypia with hyperchromasia is almost always a feature of these tumors (133,135). Epithelioid schwannomas feature epithelioid cells arranged in a sheet or cord like pattern with vacuolar spaces in a pseudoglandular pattern with no verocay bodies or palissading but with evidence of lymphoid infiltration with hyaline changes in bigger tumors (133,135). Without special staining, many schwannomas were previously misdiagnosed as gastrointestinal stromal tumors (GISTS) and GANTs, which raises questions as to its true incidence (131,135). Schwannomas are uniformly S-100 positive (133-135), and almost always positive for vimentin reactivity however they are rarely CD 34 positive and almost always negative for GFAP, Desmin, Alpha smooth muscle actin and C-kit. In this context theoretically the use of CD 117 (C-Kit) and S-100 staining should be enough to differentiate schwannomas from GISTS (131,135).
The management of anorectal schwannomas is surgical excision. A variety of surgical approaches have been reported, from abdominoperoneal resection to transrectal micro-surgical approaches, however the most appropriate procedure has not yet been defined. Controversy exists because of the submucosal nature of these lesions, making obtaining a definitive tissue diagnosis challenging and in the context of a high suspicion of malignancy wide excision is often undertaken without positive pathology. More recent studies suggest that because of the low chance of malignant transformation, the slow growing nature of these tumors and reported disease-free survival of up to 18 years, local excision with surveillance is the best course of action without adjuvant treatment if a preoperative biopsy is definitive (135,136).
Only eleven cases of anorectal schwannoma have been reported (135-144). Three of these were schwannoma cases classified as a subtype of GANT (136,140,142). There were no malignant anorectal schwannomas reported in the English literature, however, we did find two such cases in German and Italian (145,146).
Symptoms most commonly reported included constipation and tenesmus, however most schwannomas were found incidentally during imaging for unrelated reasons. These cases tended to occur later in life, with an average age of 62, although one patient was diagnosed with a schwannoma at 28. There was no gender predilection and no deaths from anorectal schwannoma were reported.
Guiding principles and outlook
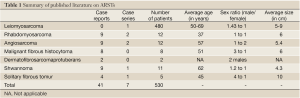
ARSTs represent a rare condition. LMSs are the most commonly identified histological type, representing 90.7% of the cases reported in this systematic review, confirming previous findings (5). Rhabdomyosarcomas are the second most common ARST in adults and the first in children (34). We did not identify a single case of liposarcoma originating from the anorectum, although there were a few retroperitoneal pelvic case reports.
Overall, we found a slightly higher incidence of ARSTs in males than in females (1.13:1), with an average age of 50.9 years and the average size at diagnosis of 5.8 cm. This is similar to the sex, age of incidence and size found in the leiomyosarcoma group and may in fact represent their numerical predominance in the cohort of patients we report (6). It is possible that more accurate diagnostic characterization of GISTs will decrease this relative predominance of LMSs. There has been significant interest in GISTs in the past decade and thoroughly documented reviews detail many aspects of this disease separately.
ARSTs present with a range of symptoms none of which are specific: constipation, diarrhea, rectal pain, tenesmus, weight loss and rectal bleed. In most cases however, ARSTs are an incidental finding on imaging done for other reasons. A complete history and physical with a rectal exam and rectoscopy is always warranted in these situations. Adequate imaging is essential and should provide specific details about location, size, homogeneity and proximity to visceral, vascular and neurological structures. A CT scan of the abdomen and pelvis is essential. Previous studies have proven that CT scans and MRI are equally adequate for soft tissue sarcomas and doing both does not improve accuracy (147). MRI should be considered if there is diagnostic uncertainty. Sigmoidoscopy or colonoscopy with a biopsy is mandatory and all patients should get a complete evaluation of the rest of their colon as many reports have been published of synchronous tumors including adenocarcinomas (148). Rectal endoscopic ultrasound (EUS) is a useful tool for biopsy if the initial sample is inconclusive, as many of these tumors are submucosal and hard to biopsy with colonoscopy. EUS can also help in distinguishing mucosal from submucosal lesion and soft tissue tumors from rectal carcinoma (149). If the mass is identified as a malignant sarcoma on pathology, a CT scan of the chest should be added. As in other locations, in case of angiosarcoma or alveolar soft part sarcoma (ASPS); cerebral metastasis is common and a CT scan of the head is also warranted (150).
Management of these cases should preferably be done in centers with expertise in both colorectal and soft tissue surgery. The rarity of these diseases warrants full imaging, pathological review (Table 2) and discussion at multidisciplinary tumor board. Some cases may require consideration of neoaduvant radio and chemotherapy, however, the literature is scant on high quality evidence to support this. Most reviews agree that post operative radiation is a relevant if margins are positive and margin re-resections are not possible or if margins are less than 1cm, although there are no clinical trials to support this approach and the use of post-operative radiation therapy should not justify poor surgery or predictable positive margins. If neoadjuvant therapy is chosen then a positron emission tomography (PET) scan would be useful in monitoring the response to chemotherapy in these patients (151).

Full table
The standard curative approach for ARSTs is surgical excision, with wide local excision (WLE) and APR being the most frequently performed interventions. Endoscopic and transanal excision should be reserved for the tumors with benign features and low local recurrence rates but are expected to play an increasing role in the future management of ARST as they become widely available and more refined. Curative resection remains the major determinant of recurrence and survival. Follow up of ARST patients should be similar to that of sarcomas of other sites with physical exam and CT imaging every 3-6 months for the first 2-3 years then every 6 months for 2 years then annually (152). Because this is a rare disease we do not expect large scale multicentre studies in the near future therefore it is advisable these patients be treated in multidisciplinary fashion in centers with colorectal and surgical oncology expertise.
Acknowledgements
Dr Meguerditchian is supported by research grants from the Cedars Cancer Institute and the Fonds de la Recherche en Santé du Québec.
Disclosure: The authors declare no conflict of interest.
References
- Horowitz J, Spellman JE Jr, Driscoll DL, et al. An institutional review of sarcomas of the large and small intestine. J Am Coll surg 1995;180:465-71.
- Walsh TH, Mann CV. Smooth muscle neoplasms of the rectum and anal canal. Br J Surg 1984;71:597-9.
- Carlier. Myoide du Rectum: Extraction. J Med Chir Pharmacol 1881;12:140-3.
- Anderson PA, Dockerty MB, Buie LA. Myomatous tumors of the rectum (leiomyomas and myosarcomas). Surgery 1950;28:642-50.
- Meijer S, Peretz T, Gaynor JJ, et al. Primary colorectal sarcoma. A retrospective review and prognostic factor study of 50 consecutive patients. Arch Surg 1990;125:1163-8.
- Hatch KF, Blanchard DK, Hatch GF 3rd, et al. Tumors of the rectum and anal canal. World J Surg 2000;24:437-43.
- Wang TK, Chung MT. Anorectal leiomyosarcomas. J Gastroenterol 1998;33:402-7.
- Feldtman RW, Oram-Smith JC, Teears RJ, et al. Leiomyosarcoma of the rectum: the military experience. Dis Colon Rectum 1981;24:402-3.
- Hishida Y, Ishida M. Smooth-muscle tumors of the rectum in Japanese. Dis Colon Rectum 1974;17:226-34.
- Minsky BD, Cohen AM, Hajdu SI, et al. Sphincter preservation in rectal sarcoma. Dis Colon Rectum 1990;33:319-22.
- Dahl I, Hagmar B, Angervall L. Leiomyosarcoma of the soft tissue. A correlative cytological and histological study of 11 cases. Acta Pathol Microbiol Scand A 1981;89:285-91.
- Miettinen M, Furlong M, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol 2001;25:1121-33.
- Brooks JJ. Immunohistochemistry of soft tissue tumors: progress and prospects. Hum Pathol 1982;13:969-74.
- Donner L, de Lanerolle P, Costa J. Immunoreactivity of paraffin-embedded normal tissues and mesenchymal tumors for smooth muscle myosin. Am J Clin Pathol 1983;80:677-81.
- Tsukada T, McNutt MA, Ross R, et al. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol 1987;127:389-402.
- Berridge DC. Leiomyosarcoma of the rectum. Report of two cases illustrating an unusual presentation and the need for repeated biopsy. Dis Colon Rectum 1987;30:721-2.
- Khalifa AA, Bong WL, Rao VK, et al. Leiomyosarcoma of the rectum. Report of a case and review of the literature. Dis Colon Rectum 1986;29:427-32.
- Quan SH, Berg JW. Leiomyoma and leiomyosarcoma of the rectum. Dis Colon Rectum 1962;5:415-25.
- Randleman CD Jr, Wolff BG, Dozois RR, et al. Leiomyosarcoma of the rectum and anus. A series of 22 cases. Int J Colorectal Dis 1989;4:91-6.
- Minsky BD, Cohen AM, Hajdu SI. Conservative management of anal leiomyosarcoma. Cancer 1991;68:1640-3.
- Minsky BD, Mies C, Rich TA. Leiomyosarcoma of the anus treated with sphincter-preserving surgery and radiation therapy. J Surg Oncol 1986;32:89-91.
- Lee YT. Leiomyosarcoma of the gastro-intestinal tract: general pattern of metastasis and recurrence. Cancer Treat Rev 1983;10:91-101.
- Adachi M, Wellmann KF, Garcia R. Metastatic leiomyosarcoma in brain and heart. J Pathol 1969;98:294-6.
- Lopez FF, Mangi A, Mylonakis E, et al. Atrial fibrillation and tumor emboli as manifestations of metastatic leiomyosarcoma to the heart and lung. Heart Lung 2000;29:47-9.
- Kusminsky RE, Bailey W. Leiomyomas of the rectum and anal canal: report of six cases and review of the literature. Dis Colon Rectum 1977;20:580-99.
- Mindich B, Tykot H, Littman L, et al. Leiomyosarcoma of the rectum: report of a case. Dis Colon Rectum 1975;18:233-6.
- Nemer FD, Stoeckinger JM, Evans OT. Smooth-muscle rectal tumors: a therapeutic dilemma. Dis Colon Rectum 1977;20:405-13.
- Neuman Z. Leiomyosarcoma of the rectum, developing from benign leiomyoma. Ann Surg 1952;135:426-30.
- Hicks J, Flaitz C. Rhabdomyosarcoma of the head and neck in children. Oral Oncol 2002;38:450-9.
- Sandberg AA, Stone JF, Czarnecki L, et al. Hematologic masquerade of rhabdomyosarcoma. Am J Hematol 2001;68:51-7.
- Stindl R, Fiegl M, Regele H, et al. Alveolar rhabdomyosarcoma in a 68-year-old patient identified by cytogenetic analysis of bone marrow. Cancer Genet Cytogenet 1998;107:43-7.
- Raney RB Jr, Crist W, Hays D, et al. Soft tissue sarcoma of the perineal region in childhood. A report from the Intergroup Rhabdomyosarcoma Studies I and II, 1972 through 1984. Cancer 1990;65:2787-92.
- Neville HL, Andrassy RJ, Lobe TE, et al. Preoperative staging, prognostic factors, and outcome for extremity rhabdomyosarcoma: a preliminary report from the Intergroup Rhabdomyosarcoma Study IV (1991-1997). J Pediatr Surg 2000;35:317-21.
- Blakely ML, Andrassy RJ, Raney RB, et al. Prognostic factors and surgical treatment guidelines for children with rhabdomyosarcoma of the perineum or anus: a report of Intergroup Rhabdomyosarcoma Studies I through IV, 1972 through 1997. J Pediatr Surg 2003;38:347-53.
- Watanabe Y, Yamaguchi A, Isogai M, et al. Treatment strategies for perianal rhabdomyosarcoma: report of two cases. Surg Today 2004;34:719-24.
- Meyer S, Kingston H, Taylor AM, et al. Rhabdomyosarcoma in Nijmegen breakage syndrome: strong association with perianal primary site. Cancer Genet Cytogenet 2004;154:169-74.
- Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073-85.
- Cho KR, Olson JL, Epstein JI. Primitive rhabdomyosarcoma presenting with diffuse bone marrow involvement: an immunohistochemical and ultrastructural study. Mod Pathol 1988;1:23-8.
- Kahn HJ, Yeger H, Kassim O, et al. Immunohistochemical and electron microscopic assessment of childhood rhabdomyosarcoma. Increased frequency of diagnosis over routine histologic methods. Cancer 1983;51:1897-903.
- Mukai M, Iri H, Torikata C, et al. Immunoperoxidase demonstration of a new muscle protein (Z-protein) in myogenic tumors as a diagnostic aid. Am J Pathol 1984;114:164-70.
- Osborn M, Hill C, Altmannsberger M, et al. Monoclonal antibodies to titin in conjunction with antibodies to desmin separate rhabdomyosarcomas from other tumor types. Lab Invest 1986;55:101-8.
- Seidal T, Mark J, Hagmar B, et al. Alveolar rhabdomyosarcoma: a cytogenetic and correlated cytological and histological study. Acta Pathol Microbiol Immunol Scand A 1982;90:345-54.
- Om A, Ghose T. Use of Anti-Skeletal Muscle Antibody from Myasthenic Patients in the Diagnosis of Childhood Rhabdomyosarcomas. Am J Surg Pathol 1987;11:272-6.
- Ishiguro N, Baba T, Ishida T, et al. Carp, a cardiac ankyrin-repeated protein, and its new homologue, Arpp, are differentially expressed in heart, skeletal muscle, and rhabdomyosarcomas. Am J Pathol 2002;160:1767-78.
- Bacon HE, Herabat T, Koohdary A, et al. Pararectal rhabdomyosarcoma: report of a case. Dis Colon Rectum 1974;17:365-9.
- Sasajima K, Okawa K, Sasamoto Y, et al. Pararectal rhabdomyosarcoma: report of a case. Dis Colon Rectum 1980;23:576-7.
- Ali R, Ozkalemkas F, Ozan U, et al. Rhabdomyosarcoma of the perianal region presenting as acute leukemia. Ann Hematol 2004;83:729-30.
- Haisfield-Wolfe ME, Rund C. A nursing protocol for the management of perineal-rectal skin alterations. Clin J Oncol Nurs 2000;4:15-21.
- Pack GT, Miller TR, Trinidad SS. Pararectal rhabdomyosarcoma: report of two cases. Dis Colon Rectum 1963;6:1-6.
- Fisher ER, Gruhn J. Perianal rhabbomyosarcoma. AMA Arch Surg 1958;77:230-4.
- Becker V, Gaa J, Ott K, et al. A rare case of primary rectal rhabdomyosarcoma in an adult. Z Gastroenterol 2006;44:1149-52.
- Roncaroli F, Montironi R, Feliciotti F, et al. Sarcomatoid carcinoma of the anorectal junction with neuroendocrine and rhabdomyoblastic features. Am J Surg Pathol 1995;19:217-23.
- Kessler KJ, Kerlakian GM, Welling RE. Perineal and perirectal sarcomas: report of two cases. Dis Colon Rectum 1996;39:468-72.
- Grobmyer SR, Clary B, Lewis JJ, et al. Adult perineal sarcomas. J Surg Oncol 2001;77:101-4.
- Marnewick J, Hulme-Moir M. Embryonal rhabdomyosarcoma of the rectum: report of a case and possible treatment option. Colorectal Dis 2010;12:e170-1.
- Meng MV, Grossfeld GD, Sudilovsky D, et al. Fine needle aspiration cytology of adult perineal rhabdomyosarcoma: a case report. Acta Cytol 2006;50:88-92.
- Okamura K, Yamamoto H, Ishimaru Y, et al. Clinical characteristics and surgical treatment of perianal and perineal rhabdomyosarcoma: analysis of Japanese patients and comparison with IRSG reports. Pediatr Surg Int 2006;22:129-34.
- Fedok FG, Levin RJ, Maloney ME, et al. Angiosarcoma: current review. Am J Otolaryngol 1999;20:223-31.
- Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer 2006;119:2922-30.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer 2009;115:4055-63.
- Falk H, Thomas LB, Popper H, et al. Hepatic angiosarcoma associated with androgenic-anabolic steroids. Lancet 1979;2:1120-3.
- Popper H, Thomas LB, Telles NC, et al. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol 1978;92:349-76.
- Ahmad A, Jamieson T, Balsitis M, et al. Radiation-induced angiosarcoma of the rectum: a case report and review of literature. Colorectal Dis 2008;10:847-8.
- Jahn SW, Liegl B, Leskowschek H, et al. Angiosarcoma at the anastomotic site mimicking local recurrence of rectal adenocarcinoma. Endoscopy 2011;43 Suppl 2 UCTN:E293-4.
- Lee JY, Oh SR, Yang BJ, et al. A Case of Angiosarcoma in Rectum. The Korean Study of Intestinal Disease 2011;9:57-60.
- Tardío JC, Nájera L, Alemany I, et al. Rectal angiosarcoma after adjuvant chemoradiotherapy for adenocarcinoma of the rectum. J Clin Oncol 2009;27:e116-7.
- Ben-Izhak O, Kerner H, Brenner B, et al. Angiosarcoma of the colon developing in a capsule of a foreign body. Report of a case with associated hemorrhagic diathesis. Am J Clin Pathol 1992;97:416-20.
- Lo YM, Gillett MB, Vina M, et al. Hemangiosarcoma of the rectum after chronic anorectal ulceration. J Clin Gastroenterol 1989;11:77-81.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol 1998;22:683-97.
- Fletcher CD, Beham A, Bekir S, et al. Epithelioid angiosarcoma of deep soft tissue: a distinctive tumor readily mistaken for an epithelial neoplasm. Am J Surg Pathol 1991;15:915-24.
- Alles JU, Bosslet K. Immunocytochemistry of angiosarcomas. A study of 19 cases with special emphasis on the applicability of endothelial cell specific markers to routinely prepared tissues. Am J Clin Pathol 1988;89:463-71.
- Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer 1982;50:970-81.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer 1996;77:2400-6.
- De Yao JT, Sun D, Powell AT, et al. Scalp Angiosarcoma Remission with Bevacizumab and Radiotherapy without Surgery: A Case Report and Review of the Literature. Sarcoma 2011;2011:160369.
- Allison KH, Yoder BJ, Bronner MP, et al. Angiosarcoma involving the gastrointestinal tract: a series of primary and metastatic cases. Am J Surg Pathol 2004;28:298-307.
- Hinterseher I, Pistorius S, Zietz C, et al. Epithelioid hemangiosarcoma of the rectum. Int J Colorectal Dis 2005;20:385-7.
- Morgan CN. Endothelioma of the Rectum. Proc R Soc Med 1932;25:1020-1.
- Norbury LE. Specimen of Endothelioma of Rectum. Proc R Soc Med 1932;25:1021-2.
- Gentry RW, Dockerty MB, Glagett OT. Vascular malformations and vascular tumors of the gastrointestinal tract. Surg Gynecol Obstet 1949;88:281-323.
- Brown CJ, Falck VG, MacLean A. Angiosarcoma of the colon and rectum: report of a case and review of the literature. Dis Colon Rectum 2004;47:2202-7.
- Patel KU, Szabo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol 2008;39:184-93.
- McArthur G. Molecularly targeted treatment for dermatofibrosarcoma protuberans. Semin Oncol 2004;31:30-6.
- Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol 2007;56:968-73.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution. Cancer 2000;88:2711-20.
- Lindner NJ, Scarborough MT, Powell GJ, et al. Revision surgery in dermatofibrosarcoma protuberans of the trunk and extremities. Eur J Surg Oncol 1999;25:392-7.
- Haycox CL, Odland PB, Olbricht SM, et al. Immunohistochemical characterization of dermatofibrosarcoma protuberans with practical applications for diagnosis and treatment. J Am Acad Dermatol 1997;37:438-44.
- Farma JM, Ammori JB, Zager JS, et al. Dermatofibrosarcoma protuberans: how wide should we resect? Ann Surg Oncol 2010;17:2112-8.
- Voth H, Landsberg J, Hinz T, et al. Management of dermatofibrosarcoma protuberans with fibrosarcomatous transformation: an evidence-based review of the literature. J Eur Acad Dermatol Venereol 2011;25:1385-91.
- Meguerditchian AN, Wang J, Lema B, et al. Wide excision or Mohs micrographic surgery for the treatment of primary dermatofibrosarcoma protuberans. Am J Clin Oncol 2010;33:300-3.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol 2010;28:1772-9.
- Kérob D, Porcher R, Verola O, et al. Imatinib mesylate as a preoperative therapy in dermatofibrosarcoma: results of a multicenter phase II study on 25 patients. Clin Cancer Res 2010;16:3288-95.
- Buche S, Delasalle EM, Coindre JM, et al. Atypical presentation of giant cell fibroblastoma. Ann Dermatol Venereol 2010;137:381-5.
- Mc Cormack O, Heaslip IS, Abdulrahim M, et al. Perianal dermatofibrosarcoma protuberans: a case report. Cutis 2011;87:85-8.
- Ozzello L, Stout AP, Murray MR. Cultural characteristics of malignant histiocytomas and fibrous xanthomas. Cancer 1963;16:331-44.
- O’Brien JE, Stout AP. Malignant Fibrous Xanthomas. Cancer 1964;17:1445-55.
- Fletcher CD. Pleomorphic malignant fibrous histiocytoma: fact or fiction? A critical reappraisal based on 159 tumors diagnosed as pleomorphic sarcoma. Am J Surg Pathol 1992;16:213-28.
- Fletcher CD. Malignant fibrous histiocytoma? Histopathology 1987;11:433-7.
- Al-Agha OM, Igbokwe AA. Malignant fibrous histiocytoma: between the past and the present. Arch Pathol Lab Med 2008;132:1030-5.
- Nahal A. Malignant Fibrous Histiocytoma: From Popularity to Obsolescence. Can J Pathol 2010;2:30-8.
- Verma P, Chandra U, Bhatia PS. Malignant histiocytoma of the rectum: report of a case. Dis Colon Rectum 1979;22:179-82.
- Levinson MM, Tsang D. Multicentric malignant fibrous histiocytomas of the colon. Report of a case and review of the subject. Dis Colon Rectum 1982;25:327-31.
- Spagnoli LG, Dell’Isola C, Sportelli G, et al. Primary malignant fibrous histiocytoma of storiform-pleomorphic type: a case report of an ano-rectal localization. Tumori 1984;70:567-70.
- Flood HD, Salman AA. Malignant fibrous histiocytoma of the anal canal. Report of a case and review of the literature. Dis Colon Rectum 1989;32:256-9.
- Singh DR, Aryya NC, Sahi UP, et al. Malignant fibrous histiocytoma of the rectum. Eur J Surg Oncol 1999;25:447-8.
- Kim BG, Chang IT, Park JS, et al. Transanal excision of a malignant fibrous histiocytoma of anal canal: a case report and literature review. World J Gastroenterol 2008;14:1459-62.
- Nakayama Y, Minagawa N, Torigoe T, et al. Malignant fibrous histiocytoma originating from the mesorectum: a case report. World J Surg Oncol 2011;9:15.
- Azizi R, Mahjoubi B, Shayanfar N, et al. Malignant fibrous histiocytoma of rectum: Report of a case. Int J Surg Case Rep 2011;2:111-3.
- Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. Am J Ind Med 1992;22:1-31.
- Hasegawa T, Hirose T, Seki K, et al. Solitary fibrous tumor of the soft tissue. An immunohistochemical and ultrastructural study. Am J Clin Pathol 1996;106:325-31.
- Bainbridge TC, Singh RR, Mentzel T, et al. Solitary fibrous tumor of urinary bladder: report of two cases. Hum Pathol 1997;28:1204-6.
- Kelly PM, Baxter GM. Solitary fibrous tumour of the prostate. Br J Radiol 1998;71:1086-8.
- Kim KA, Gonzalez I, McComb JG, et al. Unusual presentations of cerebral solitary fibrous tumors: report of four cases. Neurosurgery 2004;54:1004-9; discussion 9.
- Ng HK, Choi PC, Wong CW, et al. Metastatic solitary fibrous tumor of the meninges. Case report. J Neurosurg 2000;93:490-3.
- Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol 1998;22:1501-11.
- Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control 2006;13:264-9.
- Yousem SA, Flynn SD. Intrapulmonary localized fibrous tumor. Intraparenchymal so-called localized fibrous mesothelioma. Am J Clin Pathol 1988;89:365-9.
- England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640-58.
- Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer 2002;94:1057-68.
- Moran CA, Suster S, Koss MN. The spectrum of histologic growth patterns in benign and malignant fibrous tumors of the pleura. Semin Diagn Pathol 1992;9:169-80.
- Witkin GB, Rosai J. Solitary fibrous tumor of the mediastinum. A report of 14 cases. Am J Surg Pathol 1989;13:547-57.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Frequent expression of bcl-2 protein in solitary fibrous tumors. Jpn J Clin Oncol 1998;28:86-91.
- Renshaw AA, Pinkus GS, Corson JM. Cd34 and Ae1/Ae3-Diagnostic Discriminants in the Distinction of Solitary Fibrous Tumor of the Pleura from Sarcomatoid Mesothelioma. Appl Immunohistochem 1994;2:94-102.
- de Perrot M, Fischer S, Brundler MA, et al. Solitary fibrous tumors of the pleura. Ann Thorac Surg 2002;74:285-93.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol 1999;30:1464-73.
- Dudkiewicz M, Deschenes JL, Bloom C, et al. Solitary fibrous tumor of the ischioanal fossa: report of a case and review of the literature. Dis Colon Rectum 2004;47:535-7.
- Joe BN, Bolaris M, Horvai A, et al. Solitary fibrous tumor of the male pelvis: findings at CT with histopathologic correlation. Clin Imaging 2008;32:403-6.
- Mourra N, Lewin M, Sautet A, et al. Epithelioid solitary fibrous tumor in the ischioanal fossa. Virchows Arch 2005;446:674-6.
- Yoshida R, Takada H, Iwamoto S, et al. A solitary fibrous tumor in the perianal region with a 13-year follow-up: report of a case. Surg Today 1999;29:642-5.
- Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69.
- Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, et al. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod pathol 1998;11:728-34.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors - Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001;438:1-12.
- Chen KT, Latorraca R, Fabich D, et al. Malignant schwannoma: a light microscopic and ultrastructural study. Cancer 1980;45:1585-93.
- Herrera GA, Pinto de Moraes H, Grizzle WE, et al. Malignant small bowel neoplasm of enteric plexus derivation (plexosarcoma). Light and electron microscopic study confirming the origin of the neoplasm. Dig Dis Sci 1984;29:275-84.
- Weiss SW, Langloss JM, Enzinger FM. Value of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest 1983;49:299-308.
- Miettinen M, Shekitka KM, Sobin LH. Schwannomas in the colon and rectum: a clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol 2001;25:846-55.
- Hsu KF, Lin CT, Wu CC, et al. Schwannoma of the rectum: report of a case and review of the literature. Rev Esp Enferm Dig 2010;102:289-91.
- Abel ME, Nehme Kingsley AE, Abcarian H, et al. Anorectal neurilemomas. Dis Colon Rectum 1985;28:960-1.
- Kakizoe S, Kuwahara S, Kakizoe K, et al. Local excision of benign rectal schwannoma using rectal expander-assisted transanal endoscopic microsurgery. Gastrointest Endosc 1998;48:90-2.
- Murakami N, Tanaka T, Ohmori Y, et al. A case report of rectal schwannoma. Kurume Med J 1996;43:101-6.
- Maciejewski A, Lange D, Wloch J. Case report of schwannoma of the rectum--clinical and pathological contribution. Med Sci Monit 2000;6:779-82.
- Bhardwaj K, Bal MS, Kumar P. Rectal schwannoma. Indian J Gastroenterol 2002;21:116-7.
- Mulchandani MH, Chattopadhyay D, Obafunwa JO, et al. Gastrointestinal autonomic nerve tumours - Report of a case and review of literature. World J Surg Oncol 2005;3:46.
- Pescatori M, Brusciano L, Binda GA, et al. A novel approach for perirectal tumours: the perianal intersphincteric excision. Int J Colorectal Dis 2005;20:72-5.
- Lee SH, Kim TO, Hwang SY, et al. A case of rectal schwannoma presenting with hematochezia. Korean J Gastroenterol 2006;48:195-9.
- Reinbold WD, Hillemanns A, Seesko H, et al. Malignant schwannoma of the rectum. Radiologe 1996;36:663-6.
- Catania G, Puleo C, Cardi F, et al. Malignant schwannoma of the rectum: a clinical and pathological contribution. Chir Ital 2001;53:873-7.
- Panicek DM, Gatsonis C, Rosenthal DI, et al. CT and MR imaging in the local staging of primary malignant musculoskeletal neoplasms: Report of the Radiology Diagnostic Oncology Group. Radiology 1997;202:237-46.
- Alfonso AE, Hertz RE. Synchronous leiomyosarcoma and adenocarcinoma of the rectum: report of a case and review of the literature. Dis Colon Rectum 1975;18:228-32.
- Wolf O, Glaser F, Kuntz C, et al. Endorectal ultrasound and leiomyosarcoma of the rectum. Clin Investig 1994;72:381-4.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer 1995;75:989-96.
- Schuetze SM, Rubin BP, Vernon C, et al. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer 2005;103:339-48.
- Kane JM 3rd. Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Curr Opin Oncol 2004;16:328-32.