Phase II trial of capecitabine plus nab-paclitaxel in patients with metastatic pancreatic adenocarcinoma
Introduction
Metastatic pancreatic adenocarcinoma (mPC) is a lethal disease with a median survival of approximately 6-7 months in the gemcitabine ± erlotinib era (1). Introduction of the combination of 5-fluorouracil (5-FU), leucovorin, oxaliplatin, and irinotecan (FOLFIRINOX), as well as the better tolerated nab-paclitaxel plus gemcitabine regimen, represented a significant advance in the treatment of mPC (2,3).
Apart from the principial antitumor potential of capecitabine in advanced pancreatic cancer when given as a radiosensitizer (4) or in combination with gemcitabine (5,6) or gemcitabine + oxaliplatin (7,8), this oral 5-FU prodrug seems to represent a particularly attractive combination partner for nab-paclitaxel: taxanes upregulate thymidine phosphorylase in liver tissue, potentially increasing the tumor concentration and efficacy of capecitabine (9).
In view of the recently described excellent therapeutic index of capecitabine plus nab-paclitaxel in metastatic breast cancer (10), we initiated the present phase II trial to evaluate this combination as first-line therapy in mPC. The primary objective of the trial was to determine the objective response rate (ORR), secondary objectives included determination of the disease control rate (DCR), progression-free survival (PFS), and overall survival (OS), as well as evaluation of the safety and tolerability of this combination when administered according to an intra-individual dose escalation schedule.
The trial is registered with the European Medicines Agency as EudraCT 2013-001714-15.
Patients and methods
Patient population
Adults (≥18 years of age) with histologically or cytologically confirmed metastatic adenocarcinoma of the pancreas and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 were enrolled. Measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 was required (11). Additional eligibility criteria included adequate hepatic, hematologic, and renal function. No previous chemotherapy for metastatic disease was allowed. Adjuvant gemcitabine was permitted if the last cycle was completed ≥6 months prior to trial entry.
Trial medication
Patients received capecitabine (825 mg/m2 orally twice daily on days 1-15) and nab-paclitaxel (125 mg/m2 intravenously on days 1 and 8) every 3 weeks. In patients with adequate bone marrow function (neutrophils ≥1,500/µL, thrombocytes ≥100,000/µL) and with no clinically relevant adverse reactions [defined as adverse events (AEs), other than alopecia or fatigue, that were of ≤ grade 2 severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0] after the first cycle of treatment (11), the nab-paclitaxel dose was escalated to 100 mg/m2 on days 1, 8, and 15 of each subsequent cycle, and was maintained at this level if tolerated.
Granulocyte-colony stimulating factor (G-CSF) was recommended in case of neutropenia of grade >2. In patients with an objective treatment response or with stable disease (SD), treatment was continued until the development of progressive disease (PD). In patients who derived a clinical benefit, and in whom either capecitabine or nab-paclitaxel had to be discontinued for toxicity reasons (for example, hand-foot syndrome associated with capecitabine, or neuropathy associated with nab-paclitaxel), continuation with the non-dose-limiting component of the combination as monotherapy was recommended.
Trial design and statistical considerations
The study was conducted in accordance with the International Conference on Harmonization E6 requirements for Good Clinical Practice and with the ethical principles outlined in the Declaration of Helsinki (12).
This was a single-center, open-label, phase II clinical trial. The primary endpoint of the trial was the ORR according to RECIST version 1.1 criteria. This was evaluated at baseline and every 2 months thereafter, with assessments carried out by an independent radiological review committee. Secondary endpoints included DCR (abrogation of PD), PFS, and OS, in addition to evaluations of the safety and tolerability of capecitabine plus nab-paclitaxel in mPC. The latter was assessed by the incidence of treatment-related AEs according to NCI-CTCAE version 4.0.
In order to demonstrate that capecitabine plus nab-paclitaxel administered according to an intra-individual dose-escalation schedule would yield an ORR of ≥30% with 80% power, it was estimated that we needed to enrol 32 patients to obtain a sample size of 29 evaluable patients.
Results
Between December 2013 and January 2015, 30 patients were enrolled into this single-center, phase II clinical trial. Patients’ median age was 63 years. All patients had an ECOG PS of 0-1, most patients (93%) had multiple metastatic sites, 80% had liver metastases, and 23% had biliary stents in place at the time of trial entry. Median CA19-9 was 1,004 U/mL (0.9-100,000 U/mL). Patients’ baseline characteristics are shown in Table 1.
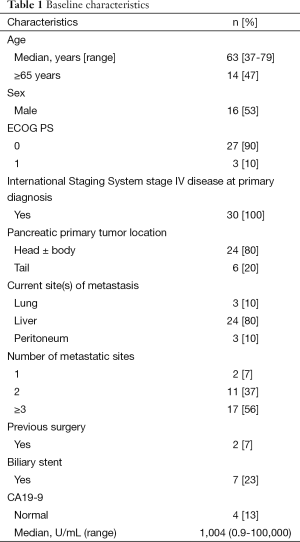
Full table
Treatment efficacy
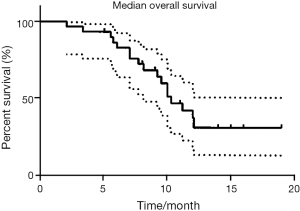
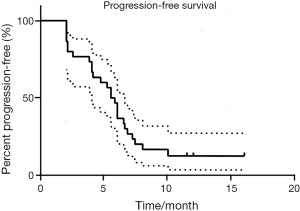
Among the 29 RECIST response-assessable patients, the ORR was 41.4%, and SD was noted in 34.5% (Table 2). This gave a DCR of 76%. As shown in Figures 1 and 2, after a median follow-up of 10.3 months (range, 1.9-19.0 months), the median PFS is 5.6 months (range, 1.9-16.0 months), and median OS is 10.3 months (range, 2.0-19.0+ months), with 13/30 (43.3%) patients remaining alive at present.
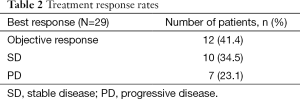
Full table
Rapid decreases in CA-19/9 levels were observed. Among patients with pathologically elevated baseline values, 24/30 (92%) had a >20% decrease, 18/30 had a >50% decrease (60%), and 9 had a >90% decrease. CA-19/9 levels were correlated with increased survival. Patients with a >90% decrease in CA-19/9 levels had a 62% ORR, and 6.7 and 11 months of PFS and OS respectively.
It should be mentioned that 2nd-line chemotherapy with gemcitabine + oxaliplatin + erlotinib (n=18) was effected upon PD in 18 (60%) of our patients. In agreement with our and others’ previous experience (13-15), half of them benefited by achieving ≥SD. Third-line treatment with FOLFIRI was given to 4 patients (13%).
Safety
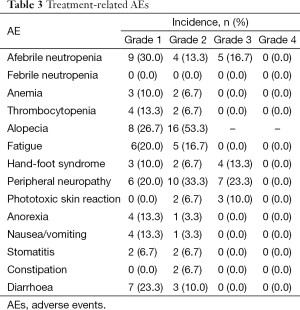
All 30 evaluable patients were included in the safety analysis. In all except two of these patients, it was possible to escalate the nab-paclitaxel dose after the first treatment cycle. A total of 180 cycles of nab-paclitaxel and 193 cycles of capecitabine were administered per protocol. A summary of treatment-related AEs by severity is presented in Table 3.
Full table
The only AEs of grade 3 severity were peripheral neuropathy (23.3%), afebrile neutropenia (16.7%), hand-foot syndrome (13%), and phototoxic skin reaction (10%). No grade 4 AEs occurred.
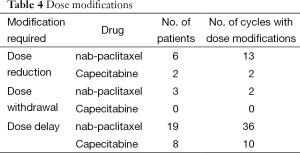
Table 4 shows the number of patients who required capecitabine or nab-paclitaxel dose modifications during the course of the trial. Two patients required capecitabine dose reductions. In each of the six patients who required nab-paclitaxel dose reductions, the dose was reduced to the starting level of 125 mg/m2 on days 1 and 8 every 3 weeks. nab-paclitaxel and capecitabine dosing delays were required by 19 and eight patients, respectively. Three patients had to discontinue nab-paclitaxel, while all 29 evaluable patients were able to remain on capecitabine for the duration of the trial.
Full table
Conclusions
The combination of capecitabine plus nab-paclitaxel shows substantial antitumor activity when administered as first-line chemotherapy in mPC: The ORR according to RECIST criteria was 41%, and the DCR (objective response + SD) was 76% After a median follow-up of 10.3 months, median PFS and median OS are 5.6 and 10.3 months, respectively with 13 patients (43%) remaining alive. The described dose regimen of capecitabine plus nab-paclitaxel can be administered safely: Intra-individual dose escalations were feasible in 28/30 patients, and could be maintained in the large majority of cases (93%). The only AEs of grade 3 severity were transient peripheral neuropathy (23%), afebrile neutropenia (17%), hand-foot syndrome (13%) and phototoxic skin reaction (10%). No grade 4 AEs occurred.
Acknowledgements
Financial support for this trial was provided by Celgene Corporation, NJ, USA. Nab-paclitaxel was supplied by Celgene Corporation, and capecitabine was supplied in part by Fresenius Kabi, Austria. Writing assistance was provided by John R. McGuire, PhD, Meditech Media, LLC. Biostatistical support was provided by Peng Wu, PhD, MS, Celgene Corporation. The authors were fully responsible for all content and editorial decisions for this manuscript.
Footnote
Conflicts of Interest: W Scheithauer—Consultant & advisory role, honoraria and research funding, Celgene Corporation; G Kornek and G Prager—honoraria as invited speakers, Celgene Corporation; S Schindl—advisory role, Celgene Corporation.
References
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [PubMed]
- Huguet F, Hammel P, Vernerey D, et al. Impact of chemoradiotherapy (CRT) on local control and time without treatment in patients with locally advanced pancreatic cancer (LAPC) included in the international phase III LAP 07 study. J Clin Oncol 2014;32:abstr 4001.
- Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513-8. [PubMed]
- Scheithauer W, Schüll B, Ulrich-Pur H, et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol 2003;14:97-104. [PubMed]
- Lim JY, Cho JH, Lee SJ, et al. Gemcitabine combined with capecitabine compared to gemcitabine with or without erlotinib as first-line chemotherapy in patients with advanced pancreatic cancer. Cancer Res Treat 2015;47:266-73. [PubMed]
- Petrioli R, Roviello G, Fiaschi AI, et al. Gemcitabine, oxaliplatin, and capecitabine (GEMOXEL) compared with gemcitabine alone in metastatic pancreatic cancer: a randomized phase II study. Cancer Chemother Pharmacol 2015;75:683-90. [PubMed]
- Ishikawa T, Utoh M, Sawada N, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new flouoropyrimidine carbamate in human cancer xenografts. Biochem Pharmacol 1998;55:1091-7. [PubMed]
- Schwartzberg LS, Arena FP, Mintzer DM, et al. Phase II multicenter trial of albumine-bound paclitaxel and capecitabine in first-line treatment of patients with metastatic breast cancer. Clin Breast Cancer 2012;12:87-93. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- Good clinical practice research guidelines reviewed, emphasis given to responsibilities of investigators: second article in a series. J Oncol Pract 2008;4:233-5. [PubMed]
- Fuereder T, Stift J, Kuehrer I, et al. Response to GEMOX plus erlotinib in pancreatic cancer is associated with ERCC1 overexpression. Eur J Clin Invest 2014;44:958-64. [PubMed]
- Demols A, Peeters M, Polus M, et al. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer 2006;94:481-5. [PubMed]
- Fortune BE, Li X, Kosuri KV, et al. Fixed-dose-rate gemcitabine in combination with oxaliplatin in patients with metastatic pancreatic cancer refractory to standard-dose-rate gemcitabine: a single-institute study. Oncology 2009;76:333-7. [PubMed]


