Metastatic colorectal cancer presenting with bone marrow metastasis: a case series and review of literature
Introduction
Despite being one of the most preventable cancers, colorectal cancer (CRC) continues to be a major public health burden worldwide, with death usually attributed to disease recurrence or spread. The distant metastases from colorectal tumors usually occur in the liver and lungs. Skeletal metastases are extremely rare, particularly when encountered in the absence of lung and liver metastases. Bone and bone marrow metastases (BMM) from CRC usually reflect a widespread disease that has been heavily treated and portend an ominous prognosis with a median survival of less than 10 months (1-7). Although these metastases can be expected in patients with disseminated solid tumors, these are seldom a presenting sign of carcinoma.
Even with paramount advances in understanding different aspects of CRC, the last few decades of research have failed to depict pathways incriminated in all routes of spread. We herein report a series of three patients who presented with isolated bone and BMM as first presentation of an underlying CRC. This paper aims to review the literature for all data on skeletal and BMM from CRC, illuminating the postulated mechanisms of spread. We recommend that physicians be aware of the possibility and the risk of this unusual phenomenon in their evaluation of patients with bony symptoms in the setting of CRC.
Case presentation
Patient 1
A 75-year-old man was diagnosed in July 2000 with stage IIIB (T3N1M0) right colon adenocarcinoma for which he underwent hemicolectomy followed by adjuvant 5-fluorouracil (5-FU) and leucovorin. Until 2010, he was regularly followed with no evidence of disease recurrence.
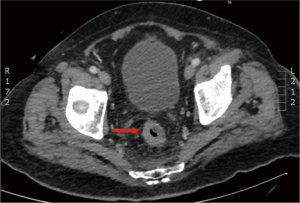
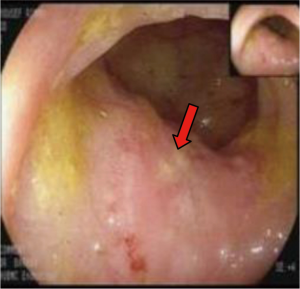
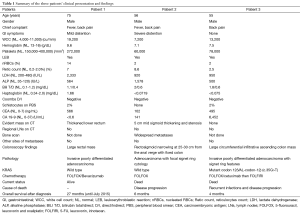
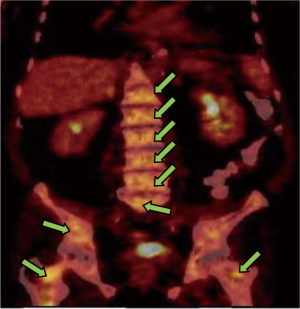
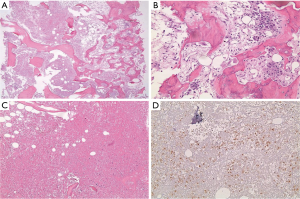
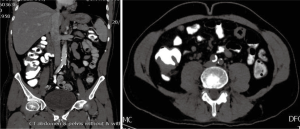
In March 2013, he presented to the American University of Beirut Medical Center (AUBMC) with 2-month history of low back pain, fevers, weight loss and mild abdominal distention. The exam showed an elderly frail man who was pale, febrile and confused. Laboratory findings were remarkable for a white cell count (WCC) of 19,200 cu/mm [normal (NL), 4,000-11,000 cu/mm], a hemoglobin (Hb) level of 9.6 g/dL (NL, 13-16 g/dL), platelets count of 272,000/mm3 (NL, 150,000-400,000/mm3); reticulocyte count of 7% (0.2-2.0%), and lactate dehydrogenase (LDH) 2,333 IU/L (NL, 200-480 IU/L) (see Table 1 for further readings). Peripheral blood smear showed moderate schistocytosis of 2% with nucleated red blood cells (nRBCs) reaching 14% and a leukoerythroblastic reaction. Subsequently, CT of chest, abdomen and pelvis (Figure 1) revealed thickened and narrowed lower rectum with no evidence of masses or lymph nodes elsewhere in the body. PET scan (Figure 2) also revealed mild increased activity in the lower rectum with diffuse abnormal activity involving the entire skeleton. A bone marrow (BM) biopsy (Figure 3) was concomitantly done and it showed more than 90% extensively necrotic malignant cells in the marrow and scattered viable hematopoietic component with cohesive clusters of large carcinomatous cells. The latter stained positive for cytokeratin 20 (CK20) and CDX-2 and negative for CK7 and PSA, suggesting a gastrointestinal origin. In view of the mentioned findings and a high serum CEA level of 566 ng/mL (NL, 0-7 ng/mL), colonoscopy followed and showed a large rectal mass (Figure 4) biopsy of which proved positive for invasive poorly differentiated adenocarcinoma. Molecular studies of the tumor revealed a wild-type KRAS gene in codons 12 and 13.
Full table
In April 2013, the patient was started on chemotherapy after clinical stabilization. The patient received 12 cycles of 5-FU, leucovorin and oxaliplatin (FOLFOX) with Bevacizumab. After 2 months of treatment, the BM biopsy of June 2013 showed less than 5% residual disease with no necrosis, and grade 2 reticulin fibrosis. The BM was free of disease by September 2013 (after 6 months of treatment). The CEA gradually dropped to normal and the patient has been kept on 5-FU, leucovorin and bevacizumab until the date of this report with complete response on serial PET scans.
Patient 2
A 56-year-old man presented to AUBMC in October 2013 for evaluation of severe back pain, fever and abdominal distention of 20 days duration. On physical examination he had slightly icteric sclera and diffuses paraspinal tenderness. The complete blood count (CBC) showed a WBC of 7,200 cu/mm (NL, 4,000-11,000 cu/mm), Hb 7.1 g/dL (NL, 13-16 g/dL), platelets count of 60,000/mm3 (NL, 150,000-400,000/mm3) and evident early myeloid precursors. The reticulocyte count was 8.6% (NL, 0.2-2.0%). Other routine laboratory tests were relevant for a total bilirubin of 2 mg/dL (NL, 0.1-1.2 mg/dL) with direct bilirubin of 0.6 mg/dL (NL, 0-0.2 mg/dL); serum haptoglobin was less than 0.0719 mg/dL (NL, 0.34-2.0 mg/dL); alkaline phosphatase was 1,578 IU/L (NL, 35-120 IU/L) and LDH of 920 IU/L (NL, 200-480 IU/L) (see Table 1 for further readings). In addition, peripheral blood smear showed leukopenia, thrombocytopenia, and rare nucleated RBCs.
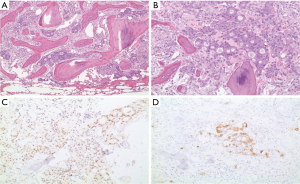
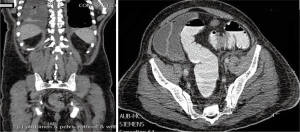
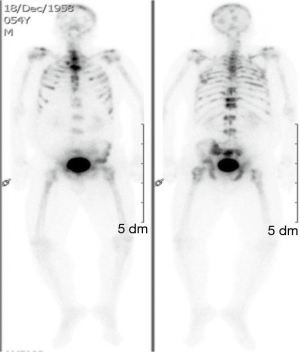
The clinical suspicion in this case was that of myeloma. On BM studies (Figure 5), the marrow was found significantly invaded by a metastatic, gland forming adenocarcinoma with focal signet ring cytology. These tumor cells stained positive for CK 7 and partially for CK 20 and CDX-2 and negative for TTF-1. This immunoprofile suggested a gastrointestinal origin. Colonoscopy (Figure 6) was then performed and showed significant rectosigmoid narrowing at 25-30 cm from the anal verge with fixed colon. No definite masses were seen and no biopsy could have been taken. Gastroscopy was normal. Enhanced CT scans of the chest, abdomen and pelvis with oral, intravenous and rectal contrast (Figure 7) showed apparent thickening of an approximately 5 cm mid sigmoid segment with secondary stenosis associated with several diverticula at that level. On the bone window, diffuse mixed sclerotic and lytic lesions were seen along the entire skeleton, highly suspicious of metastatic disease. A bone scan (Figure 8) confirmed the widespread metastatic disease involving the axial and appendicular skeleton as well as the skull. Moreover, CA 19-9 level was 141 U/mL (NL, 0-37 U/mL) and CEA 102 ng/mL (NL, 0-7 ng/mL). Molecular studies on the BM specimen for BRAF V600E, KRAS codons 12 and 13, HRAS mutations and NRAS-exons 2, 3 and 4 mutations were all negative.
The patient then received one cycle of FOLFOX as treatment of a primary CRC. Unfortunately, he was lost to follow up but we found that he continued chemotherapy outside our institution and then died of disease 6 months after initial diagnosis.
Patient 3
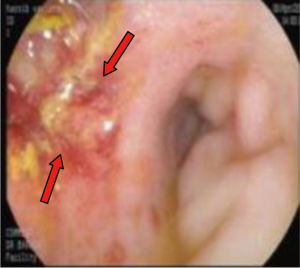
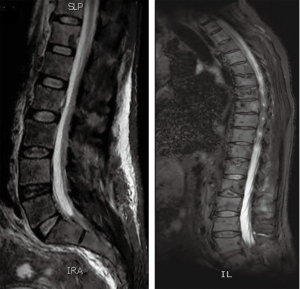
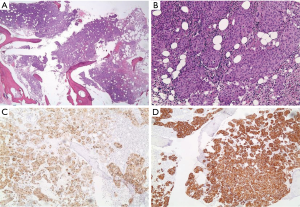
A 55-year-old man presented to AUBMC in August 2013 for evaluation of severe low back pain after sustaining a fall. He also reported having significant weight loss and decreased appetite without any gastrointestinal symptoms. A meticulous physical exam failed to disclose any relevant findings. Upon presentation, the CBC showed a WBC of 13,200 cu/mm (NL, 4,000-11,000 cu/mm), a Hb of 7.5 g/dL (NL, 13-16 g/dL), platelets count of 78,000/mm3 (NL, 150,000-400,000/mm3) with some early myeloid precursors and nRBCs of 2%. The reticulocyte count was slightly elevated at 2.5% (0.2-2.0%) and the serum haptoglobin was very low, less than 0.075 mg/dL (NL, 0.34-2.0 mg/dL) (see Table 1 for further readings). The LDH recorded a high level of 950 IU/L (NL, 200-480 IU/L) and the peripheral blood smear was remarkable for moderate schistocytes. Owing to the history of fall and the presence of back pain, a magnetic resonance imaging (MRI) of the whole spine (Figure 9) was performed showing a patchy abnormal signal involving multiple vertebral bodies of the thoracic and dorsal spine with complete involvement of D4 vertebra. An intradural and extramedullary lesion was also found at L5 level with abnormal soft tissue thickening, having increased T1 signal intensity and bright signal on T2-weighted images. These findings were highly suggestive of infiltrative malignant disease. BM studies (Figure 10) showed a metastatic carcinoma forming sheets and nests with focal glandular formation, mucin production and necrosis, replacing about 80% of marrow cellularity. These tumor cells stained positive for CK 7 and partially for CK 20 and CKA1/3, and CK8/18 while negative for TTF-1, HEPAR-1, PSA and Synaptophysin. The differential diagnoses included carcinomas of the pancreas, bile duct, urothelium and stomach. Moreover, the Ca 19-9 was markedly increased at 6,452 U/mL (NL, 0-37 U/mL) and CEA at 495 ng/mL (NL, 0-7 ng/mL). Enhanced CT scans of the chest abdomen and pelvis with oral and intravenous contrast (Figure 11) only showed a slightly prominent liver measuring 18 cm of size with homogeneous CT density but no evidence of pancreatic masses or biliary ductal dilatation. On the bone window, lytic lesions with compression fractures involving the vertebral bodies of T8, T9, T12, L1 and L2 were apparent. A bone scan was not performed. Colonoscopy (Figure 12) showed a large circumferential infiltrative ascending colon mass, biopsy of which revealed an invasive poorly differentiated adenocarcinoma with signet ring features. KRAS gene was found mutated at codon 12 (VAL-Codon-12) (c.35G>T). Despite four cycles of FOLFOX, his disease significantly progressed and the hospital course was complicated by recurrent bacteremia. Unfortunately, he died in hospital 4 months after the initial diagnosis.
Discussion
CRC is the third leading cause of cancer-related death in the world (8) for both men and women. Similar to most other malignancies, CRC has historically established a certain pattern of metastasis. More than 70% of patients will have liver involvement as the most common first site of spread. However, in 40-50% of these patients, other organs are concomitantly involved by colorectal metastases. The lung is the second most common target organ, hosting 20-30% of all distant CRC metastases (9). In contrast, metastases to adrenals, bones, brain and skin have seldom been reported.
The breakthroughs in understanding CRC pathogenesis, management and treatment have resulted in marked improvement in the overall survival even in metastatic patients. Therefore, the likelihood of developing rare and symptomatic metastases during the course of the disease has clearly increased in parallel.
Skeletal metastases of primary CRC appear to be more frequently detected, incidentally or purposely, especially with improved radiological tools and the expanding role of PET scan. However, a thorough literature review hardly reports an overall incidence of 5-11% (1-3,10,11). Since bony metastases are most often associated with synchronous liver and lung metastases, isolated skeletal metastases are even more uncommon, occurring at an estimated frequency of 1-2% (11). Despite the increased awareness of their existence, the literature about the natural history is scarce (12) and reported studies focused primarily on determining prognostic variables in relation with metastasis to other organs (1,11,13-15).
Review of reported studies (see Table 2)

Full table
In a study by Sundermeyer et al. (16), 1,020 patients with metastatic CRC were collected from 1993 to 2002 at the Fox Chase Cancer Center and their medical records were retrospectively reviewed. The incidence of bone and brain metastases was found to be 10.4% and 3% respectively. In this study, the presence of lung metastases was the most important predictive factor of development of bone metastases. The skeletal metastases were also more common with increased numbers of active systemic agents received: 0 agent (3.7%), 1 (9.4%), 2 (10.9%), 3 (16.3%), and 4 or 5 agents (17.4%; P=0.001; trend test). Interestingly, those who received Irinotecan or Oxaliplatin were significantly more likely to develop bone metastases (13.2% vs. 8.3%, P=0.01 for Irinotecan; 16.9% vs. 9%, P=0.003 for Oxaliplatin). Regarding the location of the tumor, patients with primary rectal versus primary colon were more likely to have bone metastases (16% vs. 8.6%; P=0.001) at any point of time.
Kanthan et al. (1) also studied this pattern in a population-based retrospective survey covering a 25-year review of 5,352 patients with skeletal metastases secondary to CRCs registered at The Saskatchewan Cancer Foundation in Canada from 1970 to 1995. In this report, the incidence of osseous metastases was found to be 6.6%, in correlation with the reported literature. However, among these patients (6.6%), 17% had skeletal metastases only, concluding to a 1.1% frequency of isolated metastases, whereas 83% had skeletal metastases in combination with lung, liver or brain metastases.
Interestingly, Kanthan et al. (1) found that the disease-free interval from the time of diagnosis of CRC to the onset of skeletal metastases ranged from 10 days to 5,309 days, with 38% of patients with skeletal metastases being alive at 5 years of follow up, compared to 16% only in cases of skeletal and other metastases. However, there was no significant difference in the 10-year survival curves from the onset of osseous metastases in the two groups.
More recently, some of the findings by Kanthan et al. were questioned by a survey conducted by Roth et al. (6) This was also a qualitative retrospective study aiming specifically at determining which site of metastasis of primary CRC more significantly predicts the development of bone lesions, and whether the presence of liver vs. lung metastases correlates better with the increased likelihood and the timing of bone metastasis. The 252 collected CRC patients were initially staged or restaged using whole-body 18F-FDG PET and CT or PET/CT in contrast to the population of Kanthan et al. study where skeletal lesions were diagnosed by a bone scan or plain radiography or both (1).
During the survey period, Roth et al. (6) again calculated an overall incidence of skeletal metastases at 5.5%. Nevertheless, no case of isolated bone metastasis was recorded. Among patients with skeletal metastases, 57% had concomitant liver involvement whereas 71% had lung metastases.
This finding contrasts with the previous study by Kanthan et al. (1) in which 1.1% of CRC population developed isolated bone metastases. One possible explanation is that Kanthan et al. (1) detected these lesions using bone scans and plain radiography, which, both, may overestimate the actual incidence of bone metastases due to their low specificity. In fact, the more sensitive and specific FDG-PET and CT scans may have contributed to early detection of liver and lung metastases compared with traditional radiography, probably accounting for the strong correlation among lesions to the liver, lung, and bone in the study of Roth et al. (6).
In summary, for Roth et al. (6) a general temporal pattern of CRC spread does truly exist: lung lesions appear to be a forerunner of future bone metastasis as evidenced by the short time span from lung metastasis to bone involvement [3.3 months (±4.2); CI, 0.7-5.9] (6).
Finally, it appears from the study of Sundermeyer et al. (16) that some difference exists between colon and rectal cancers in terms of frequency of skeletal metastases where rectal cancers are more frequently (16%) associated with bone metastases than other portions of the colon (8.6%). In addition, CRC with signet ring features have historically shown a trend toward higher incidence of bone metastasis (2,11,18). However, this finding was rejected by Santini et al. (17) who conducted a study to illustrate the natural history and characteristics of bone metastases in CRC patients and its relation to disease outcomes. Their retrospective, Italian multicenter, observational review comprised 264 patients with the majority having pathologic T3/4 disease at CRC diagnosis. The vertebral column was involved in 65% of patients, followed by hip/pelvis (34%) then long bones (26%) and other sites (17%). The median time from CRC diagnosis to development of bone metastases was 11 months, and these patients survived for a median of 7 months after bone metastases diagnosis, keeping in line with published literature. In addition, of the subsets of univariate analyses exploring tumor site, tumor histology, tumor stage, tumor grade, node status, and use of adjuvant chemotherapy as possible prerequisites for development of bone metastases, only tumor grade has been found to significantly correlate with the time to developing bone metastases (Table 2).
Possible mechanisms of skeletal metastases in CRC
It is universally accepted that isolated skeletal metastases are a common finding in breast, lung, and prostate cancers (19). By contrast, CRC typically metastasizes to bone in less than 10% of patients (11) and isolated skeletal metastases are found in almost 1% of cases (1), suggesting again that this neoplasm possesses a unique clinical and biological behavior in terms of metastatic pathways. The fact that CRC involves the liver and lungs more than other organs has been attributed to the pattern of blood flow from the colorectal area to the portal system. In addition, molecular signaling and biological interaction between organs appear to play a pivotal role in CRC distant spread (9,20,21).
Chambers et al. (20) previously demonstrated that the liver and the lungs contain dense capillary networks that trap and seed colorectal tumor cells. Furthermore, Schlüter et al. (9) conducted an experimental study on rats injected with CRC cells with different metastatic potentials. For the first time, they were able to demonstrate that each of these organs is also supported by a unique microenvironment, including vessel walls, which modulates tumor cells via inherent molecular signaling pathways and exerts effects on adhesion molecules, contributing to the efficacy of tumor spread and implantation (9,20).
The discrepancy between reported studies concerning the incidence of skeletal metastases might subsequently flow from two possible explanations: on the one hand, the microscopic metastatic disease may be present since diagnosis and it remains inactive for long periods, as a specific crosstalk between the tumor and its micro-environment and a number of particular steps are required for the formation of these secondary tumors (20). On the other hand, many patients with tumor deposits on the bones will probably never develop an overt clinical metastatic disease in these areas (16) or the process might take a certain time during the natural history of CRC. Further studies at the molecular level are however needed to clarify the pathogenesis of metastases in rarer organ sites including the skeleton.
Characteristics of skeletal metastases
The vertebral column is the most common site for osseous metastases from CRC. Other sites of spread including the skull, pelvis, sacrum, femur and humerus have been also reported (22). These sites are classically involved concomitantly or directly after visceral and lymph nodes involvement by CRC (1,23-25). However, isolated involvement of the skeleton has been reported in the English literature (1,21,26-29).
Skeletal metastases of CRC, mainly to the vertebral column, are primarily blood-borne. The paravertebral venous plexus of Batson is thought to be responsible for spread as its valveless ramifications infiltrate and invest the sacrum, the lumbar spine, and the adjacent wings of the ilia (30). In addition, the communications between the spinal veins in the lumbar region with the iliofemoral venous system may indicate that colorectal tumor emboli might seed to the lower extremities in a retrograde fashion (31).
Malignant cells may also spread after breakage of the lymphatic system. If these lymphatics do not particularly belong to the portal system, CRC cells may more readily reach to the lungs and bones (11). Finally, metastases from colorectal area to the appendicular skeleton may potentially occur through the arterial system (1).
Regarding the metastases to limbs, Chang et al. (32) suggested that the crosstalk between several factors such as trauma, temperature gradients, hormonal factors, local hemodynamic factors or immune factors, as well as the properties inherent to the metastasizing cell contribute altogether to growth of deposited CRC cells.
From bone to bone marrow (BM)
The rich vascularization of the BM makes it one of the most relevant factors accounting for the development of bone metastases. The slow blood flow in the red marrow may aid in the deposition of metastatic cells (33). In addition, the adhesive interactions between tumor cells and BM stroma activate and release a number of growth factors. These factors operate primarily during bone resorption and contribute to angiogenesis, providing a unique nutritional support for tumor growth. Therefore, the BM has been progressively recognized as a common homing repository for metastatic carcinomas, independent of the primary tumor site and the temporal pattern of metastases (34).
Metastatic CRC may leave tumor deposits on the cancellous or the cortical bone. These are usually osteolytic or mixed osteolytic/osteoblastic (1,29,35). Metastases might disrupt the normal architecture of a bone, cause severe pain, or even pathologic fractures and other threatening skeletal-related events. BMM of primary CRC is often an unnoticed event in clinical practice and is not routinely investigated. The extreme rarity of BMM as first presentation of a primary CRC stems from two possible reasons: on one hand, the BM is seldom the overt isolated site of distant involvement by any malignant disease; on the other hand, the value of BM studies is limited unless supported by some other clinical findings, such as a leukoerythroblastic reaction or abnormal peripheral blood counts.
A thorough literature review disclosed one extensive report by Weiss et al. (36). on the frequency of BMM in the setting of CRC. This was a retrospective study over 40 years investigating full necropsies of 1,541 patients. The reported rate of BMM was surprisingly higher than expected: it ranged from 16% in cases of liver metastasis with other organs invasion, to 34% in cases of liver and lung metastasis with invasion of other organs. The major study drawback is its reliance on necropsy series; the published numbers should therefore be regarded as estimates and not necessarily illustrative of the entire spectrum of BMM of primary CRC. Of note, the literature regarding the frequency of BM micro-metastases specifically in CRC patients is scarce and its clinical relevance is yet to be validated in larger studies (37-39).
Imaging the bone and bone marrow metastases (BMM)
For decades, conventional imaging modalities such as plain radiographies, bone scintigraphy, CT scan and MRI have been used to assess for the presence of bone and BMM.
Plain radiographies are not used in first place to detect metastatic bone disease since lesions might not appear if the bone mineral density is below 30-50%. However, they seem useful in the characterization of known lesions or in lesions at risk of impending fracture. Another utility of comparative plain films is the assessment of response to treatment by determination of progression of a lytic lesion into different stages of sclerosis.
Tc-99m bone scintigraphy (40) has traditionally been accepted as methodology of choice for diagnosis of osseous metastases. It certainly offers higher sensitivity than plain X-rays for detection of metastases, by simultaneously assessing the entire skeleton, staging different lesions and evaluating the response to treatment. Nevertheless, lytic lesions might not show on scintigraphy due to lack of osteoblastic reaction in some solid tumors (41).
CT scan is particularly useful in the evaluation of the vertebral column and the pelvis. It also complements plain radiographies by providing more information on the cortical involvement and the possibility of an imminent fracture. CT scan can as well guide biopsies of culprit bony lesions or the BM if a trephine biopsy is not desired or feasible. However, CT scan and bone scintigraphy share a similar drawback where lesions confined to BM will not be detected due to absence of reactive bone formation or cortical destruction in neighboring bone.
Lastly, MRI has proved to be a highly sensitive tool for the detection of osseous metastases as well as highly specific for the characterization of these lesions especially when a risk of medullary or neurovascular compression exists. For the detection of BMM, MRI is very sensitive in comparison to bone scintigraphy since malignant deposits in the BM can be easily identified before reactive changes have even occurred (42). However, MRI is not specific enough in defining BMM owing to some confounding benign entities such as hemangiomas, inflammatory processes, and post-therapy changes (43,44).
In the recent years, FDG PET/CT offered a potential advantage of evaluating primary tumor site, lymph nodes status, and distant metastases, all in one study. In addition, FDG-PET has proved meticulous in detecting skeletal metastasis as well as distinctively assessing the BM for primary disease or metastatic deposits, therefore prompting accurate BM biopsies especially in cases of lymphomas and multiple myeloma (45,46). Since it relies on the high glucose metabolism of cancerous cells, PET imaging detects unsuspected metastatic lesions earlier than anatomic modifications and bone remodeling. From this fact stems the ability of PET/CT to easily identify the early changes in both medullary and cortical portions of bones whereas CT images detect cortical changes only (47). However, benign BM hyperplasia for example secondary to anemia might mask the presence of small metastatic lesions in the marrow.
In the study of Daldrup-Link et al. (48), FDG PET, whole-body MRI, and skeletal scintigraphy have a sensitivity of 90%, 82% and 71% respectively, for the detection of skeletal metastases.
More recently, BM involvement has gained major focus in imaging bony lesions in an attempt at early detection of involvement and translation into algorithms of patient management (49). A small Turkish retrospective review of 68 patients by Selcukbiricik et al. (47) studied the ability of FDG PET/CT scan to predict suspected or unsuspected BMM from the CBC, altering then the future management of these patients. PET/CT was found to have been ordered in 10 out of 68 (6.8%) patients at cancer diagnosis and out of these, 2 patients had CRC. PET/CT showed bone and BMM in 4 patients (40%) and the rest (6 patients) had BMM without bone involvement. Five patients (50%) who had probable BM involvement on their FDG PET/CT scans had unsuspected CBCs with regard to BMM. The paper concluded that PET/CT has the ability to visualize the metabolic activity of CRC cells disseminated in the BM, proving then useful as early indicator of spread.
Treatment and prognosis
The management of osseous metastases is usually directed toward palliation and involves the combination of surgery (when a single metastasis is deemed resectable) chemotherapy and radiotherapy for painful spots (1).
The prognosis of CRC patients with skeletal metastases appear to be ominous and clearly worse than other primary cancers with bony metastases (Table 2). The average 5-year survival rate of these patients is around 8.1% (4) and median survival is usually less than 10 months (3). Of the reported studies, Kanthan et al. (1) found that 38% of patients with bone vs. 16% of those with bone and visceral metastases were alive at 5 years of follow-up. Nozue et al. (2) reported a median interval of 16.5 months from the initial diagnosis and operation to the onset of osseous metastases (range, 0-108 months). The 1-year survival rate was 20% and the patients with solitary osseous metastasis survived more than 1 year. Finally, for Roth et al. (6), an average of 67% of those who developed bone involvement during the survey were dead 16 months after detection of bone metastasis.
In our report, the first case describes the development of isolated metastatic bone disease of colorectal origin 13 years after the initial diagnosis and treatment of a primary CRC. The colorectal tumor that was recently diagnosed is thought to have appeared de novo and probably entails a different biological behavior in terms of aggressiveness and metastatic potential.
Conclusions
As the worldwide CRC population is aging owing to remarkable advances in management and treatment of the disease, there might be a marked increase in recording rare organ metastases. Nevertheless, the hypotheses that CRC do not usually evolve with a primary extension to the bone and that liver and lung metastases are an ultimate prerequisite remain practically questionable. Though rare, BMM of CRC appears as a relatively early phenomenon, at least at the microscopic level. The presence of clinical marrow involvement, especially with compromised peripheral blood counts, limits the oncologist in customizing chemotherapy options and imposes a challenge of optimal dosing. Since skeletal metastases of a primary CRC have been accepted as eventual sign of impending death, early vigilant diagnosis is therefore of paramount importance in future management and treatment decisions. Better understanding of the natural history of these rare metastases may prove the existence of a different clinical and biological behavior pattern in the setting of a primary CRC. We highly encourage advanced molecular and genetic studies on this subset of patients, in the hope of tracing a particular tumor expression signature for early diagnosis, as well as improvement of survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kanthan R, Loewy J, Kanthan SC. Skeletal metastases in colorectal carcinomas: a Saskatchewan profile. Dis Colon Rectum 1999;42:1592-7. [PubMed]
- Nozue M, Oshiro Y, Kurata M, et al. Treatment and prognosis in colorectal cancer patients with bone metastasis. Oncol Rep 2002;9:109-12. [PubMed]
- Bonnheim DC, Petrelli NJ, Herrera L, et al. Osseous metastases from colorectal carcinoma. Am J Surg 1986;151:457-9. [PubMed]
- O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96:1420-5.
- Patanaphan V, Salazar OM. Colorectal cancer: metastatic patterns and prognosis. South Med J 1993;86:38-41. [PubMed]
- Roth ES, Fetzer DT, Barron BJ, et al. Does colon cancer ever metastasize to bone first? a temporal analysis of colorectal cancer progression. BMC Cancer 2009;9:274. [PubMed]
- Delva R, Pein F, Lortholary A, et al. Bone metastases of colorectal cancers: apropos of 8 cases. Rev Med Interne 1993;14:223-8. [PubMed]
- Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008;67:253-6. [PubMed]
- Schlüter K, Gassmann P, Enns A, et al. Organ-Specific Metastatic Tumor Cell Adhesion and Extravasation of Colon Carcinoma Cells with Different Metastatic Potential. Am J Pathol 2006;169:1064-73. [PubMed]
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74-85. [PubMed]
- Katoh M, Unakami M, Hara M, et al. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol 1995;30:615-8. [PubMed]
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-94. [PubMed]
- Cayla J, Rondier J, Forest M, et al. Bone metastases of colonic and rectal neoplasms. Apropos of 11 cases. Sem Hop 1975;51:507-18. [PubMed]
- Liang H, Wang XN, Wang BG, et al. Prognostic factors of young patients with colon cancer after surgery. World J Gastroenterol 2006;12:1458-62. [PubMed]
- Nikolaidis D. Skeletal metastases from carcinoma of the colon. J Maine Med Assoc 1968;59:155-8. [PubMed]
- Sundermeyer ML, Merepol NJ, Rogatko A, et al. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer 2005;5:108-13. [PubMed]
- Santini D, Tampellini M, Vincenzi B, et al. Natural history of bone metastasis in colorectal cancer: final results of a large Italian bone metastases study. Ann Oncol 2012;23:2072-7. [PubMed]
- Besbeas S, Stearns MW Jr. Osseous metastasis from carcinoma of the colon and rectum. Dis Colon Rectum 1978;21:266-8. [PubMed]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93. [PubMed]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563-72. [PubMed]
- Kuo TH, Kubota T, Watanabe M, et al. Liver colonization competence governs colon cancer metastasis. Proc Natl Acad Sci USA 1995;92:12085-9. [PubMed]
- Rodrigues J, Ramani A, Mitta N, et al. A Rare Case of Colon Cancer with Metastases to the Bone with Review of the Literature. The Internet Journal of Oncology 2012;8.
- Hoehn JL, Ousley JL, Avecilla CS. Occult carcinoma of the colon and rectum manifesting as osseous metastasis. Dis Colon Rectum 1979;22:129-32. [PubMed]
- Ihle PM, McBeath AA. Bone metastasis from colonic carcinoma. A case report. J Bone Joint Surg Am 1973;55:398-400. [PubMed]
- Patel NN, Shah PR, Wilson E, et al. An unexpected supraclavicular swelling. World J Surg Oncol 2007;5:90. [PubMed]
- Chalkidou AS, Boutis AL, Padelis P. Management of a Solitary Bone Metastasis to the Tibia from Colorectal Cancer. Case Rep Gastroenterol 2009;3:354-9. [PubMed]
- Decker FH, Fash JC. Carcinoma of rectum with metastasis to tibia. Med Radiogr 1950;26:125-8. [PubMed]
- Creedon F. Solitary peripheral bone metastasis from carcinoma of the rectum. Br J Surg 1966;53:999-1001.
- Onesti JK, et al. Isolated metastasis of colon cancer to the scapula: is surgical resection warranted? World J Surg Oncol 2011;9:137. [PubMed]
- Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg 1940;112:138-49. [PubMed]
- Libson E, Bloom RA, Husband JE, et al. Metastatic tumours of bones of the hand and foot. Skeletal Radiol 1987;16:387-92. [PubMed]
- Chang HC, Lew KH, Low CO. Metastasis of an adenocarcinoma of the stomach to the 4th metacarpal bone. Hand Surgery 2001;6:239-42. [PubMed]
- Brookes M. Blood vessels in bone marrow. In: Brookes M (ed) The blood supply of bone. An approach to bone biology. Butterworth, London, 1971; 67-91.
- Alix-Panabières C, Riethdorf S, Pantel K. Circulating Tumor Cells and Bone Marrow Micrometastasis. Clin Cancer Res 2008;14:5013-21. [PubMed]
- Gonzalez M, Peters S, Wang Y, et al. Management pulmonary metastases: when operate? Rev Med Suisse 2012;8:1326-31. [PubMed]
- Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: An analysis of 1541 necropsies. J Pathol 1986;150:195-203. [PubMed]
- Schoppmeyer K, Frühauf N, Oldhafer K, et al. Tumor cell dissemination in colon cancer does not predict extrahepatic recurrence in patients undergoing surgery for hepatic metastases. Oncol Rep 2006;15:449-54. [PubMed]
- Schott A, Vogel I, Krueger U, et al. Isolated tumor cells are frequently detectable in the peritoneal cavity of gastric and colorectal cancer patients and serve as a new prognostic marker. Ann Surg 1998;227:372-79. [PubMed]
- Weitz J, Kienle P, Magener A, et al. Detection of disseminated colorectal cancer cells in lymph nodes, blood and bone marrow. Clin Cancer Res 1999;5:1830-36. [PubMed]
- Galasko CS. Diagnosis of skeletal metastases and assessment of response to treatment. Clin Orthop 1995.64-75. [PubMed]
- Ak I, Sivrikoz MC, Entok E, et al. Discordant findings in patients with non-small-cell lung cancer: absolutely normal bone scans versus disseminated bone metastases on positron-emission tomography/computed tomography. Eur J Cardiothorac Surg 2010;37:792. [PubMed]
- Evans AJ, Robertson JF. Magnetic resonance imaging versus radionuclide scintigraphy for screening in bone metastases. Clin Radiol 2000;55:653; author reply 653-4. [PubMed]
- Altehoefer C, Blum U, Bathmann J, et al. Comparative diagnostic accuracy of magnetic resonance imaging and immunoscintigraphy for detection of bone marrow involvement in patients with malignant lymphoma. J Clin Oncol 1997;15:1754-60. [PubMed]
- Steinborn MM, Heuck AF, Tiling R, et al. Whole-body bone marrow MRI in patients with metastatic disease to the skeletal system. J Comput Assist Tomogr 1999;23:123-9. [PubMed]
- Moog F, Bangerter M, Kotzerke J, et al. 18-F-fluorodeoxyglucose-positron emission tomography as a new approach to detect lymphomatous bone marrow. J Clin Oncol 1998;16:603-9. [PubMed]
- Schirrmeister H, Bommer M, Buck AK, et al. Initial results in the assessment of multiple myeloma using 18F-FDG PET. Eur J Nucl Med Mol Imaging 2002;29:361-6. [PubMed]
- Selcukbiricik F, Yildiz O, Yilmaza S, et al. Increasing Role of FDG-PET/CT in Detecting Bone Marrow Metastasis of Solid Tumors in Adults: An Analysis of Ten Patients. World J Oncol 2012;3:271-9.
- Daldrup-Link HE, Franzius C, Link TM, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol 2001;177:229-36. [PubMed]
- Nakamoto Y, Cohade C, Tatsumi M, et al. CT Appearance of Bone Metastases Detected with FDG PET as Part of the Same PET/CT Examination. Radiology 2005;237:627-34. [PubMed]