Leptomeningeal metastasis as initial manifestation of signet ring colorectal adenocarcinoma: a case report with review of literature
Introduction
Leptomeningeal carcinomatosis (LMC) is defined as seeding of the meninges and the subarachnoid space by malignant cells. It is a rare event but is nowadays increasingly detected in association with advanced breast cancer (1), lung cancer (2) and melanomas (3-5).
Gastrointestinal cancers are responsible for 4% to 14% of cases of LMC (6). Colorectal carcinoma (CRC) spreading to the leptomeninges (7), particularly of signet ring cell origin, is even a rarer occurrence.
We herein describe an unusual case of isolated LMC in a 54-year-old man who presented with bilateral deafness as the initial symptom of a recurrent colon cancer. Brain magnetic resonance imaging (MRI) with gadolinium showed leptomeningeal enhancement suspicious of malignant spread and associated hydrocephalus. Cerebrospinal fluid (CSF) cytology had to be repeated twice to confirm the diagnosis. The patient received 10 cycles of palliative whole brain radiation therapy. However, his condition rapidly deteriorated and he died within 4 weeks of diagnosis.
Case presentation
A 54-year-old male presented to AUBMC in November 2013 with an abrupt onset of bilateral hearing loss, ataxia and altered mental status. The decreased hearing started in the right ear and was associated with headaches, progressing within days to affect both ears. He was diagnosed with stage III (T4N1M0) signet ring-cell sigmoid cancer in November 2012 for which he underwent left hemicolectomy with lymphovascular invasion present on final pathology, followed by 6 months of adjuvant FOLFOX chemotherapy (5FU-Leucovorin-Oxaliplatin) finished in April 2013.
Upon presentation the physical examination showed a fit man with normal examination of the heart, lungs and abdomen. On neurological evaluation, he was found to have right facial weakness, decreased hearing bilaterally, right-sided motor weakness, decreased plantar reflexes and an ataxic gait. The rest of the exam was normal.
The admission blood results revealed a leukocyte count of 9,900 cu/mm (normal: 4,000-11,000 cu/mm) with a normal differential, hemoglobin 15.1 g/dL (normal: 13.0-18.0 g/dL), hematocrit 43% (normal: 40.0-54.0), platelet count 163,000 cu/mm (normal: 150,000-400,000 cu/mm). The metabolic panel including chemistry, liver function tests, bilirubin levels and coagulation profile, was all within limits of normal readings. His carcinoembryonic antigen (CEA) level was slightly elevated at 5.3 ng/mL (normal: 0.0-4.0 ng/mL).
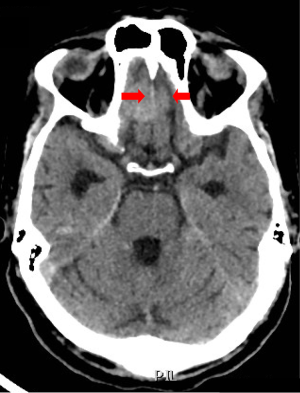
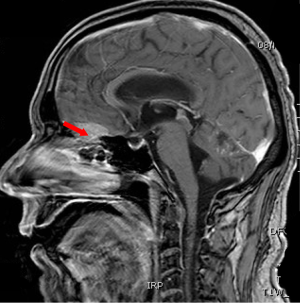
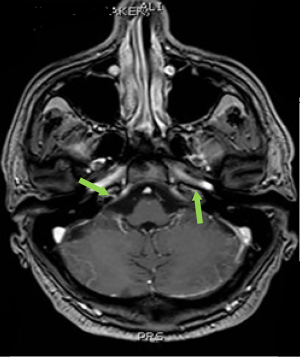
Computed tomography (CT) scan of the brain without contrast was performed to primarily rule out intracranial bleeding or any mass effect. However, it showed a 1.2 cm × 2 cm cribriform plate extra-axial tumor (Figure 1) with suspicious bilateral internal auditory canal tumors. Subsequently, brain MRI with gadolinium was done and showed enlarged ventricles out of proportion to the size of the sulci with evidence of CSF seepage. In addition, there were foci of high signal within the white matter and along the cerebral sulci and cerebellar folia indicating leptomeningeal spread causing a secondary communicating hydrocephalous. A meningeal dural lesion was also detected along the right cribriform plate that showed enhancement post-gadolinium administration measuring approximately 2.4 cm × 2.7 cm × 1.6 cm. The lesions within the internal auditory canal that had been described on the CT scan now showed evidence of enhancement on brain MRI (Figures 2,3).
In view of this picture and the high suspicion of LMC, a lumbar puncture was performed. The CSF was clear and colorless. It had a pH of 8.7, high white blood cell count of 10 cu/mm (1 neutrophil, 47 lymphocytes, 1 monocyte), no red blood cells, a high glucose level of 87 mg/dL, an elevated protein level of 0.52 g/L and some reactive mesothelial cells. The bacterial culture, tuberculosis PCR and the Brucella direct titers were all negative. The cytology was suspicious for malignant cells. Therefore, a second lumbar puncture was performed to ascertain the diagnosis. The CSF was acellular but had an elevated protein level of 0.66 g/L and a high CEA level of 231.6 ng/mL. Cytology confirmed the finding of signet ring carcinoma of colorectal origin.
CT scan of chest, abdomen and pelvis with oral and intravenous contrast showed no evidence of disease spread elsewhere in the body.
In view of the ominous prognosis, the case was thoroughly discussed with the family who opted for palliative whole brain radiotherapy (RT) along with dexamethasone for symptomatic relief. No intrathecal chemotherapy was given. Nevertheless despite a completed course of RT, the patient’s neurological status deteriorated very rapidly, he developed recurrent seizures, further cranial nerve defects, and died 6 weeks after presentation.
Discussion
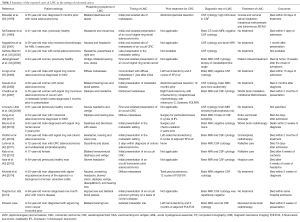
LMC was originally described by Eberth in 1870 (8). It is defined as diffuse or multifocal seeding of the leptomeninges, the subarachnoid space and the CSF by neoplastic cells (9). This metastatic involvement is a well-recognized phenomenon in association with hematologic malignancies, but is often a late and rare event complicating 1-5% of patients with solid tumors (10,11). However, this entity is thought to be underdiagnosed since necropsy studies of cancer patients have shown a higher incidence of 8% (12) to 19% (13). This rising diagnosis of LMC can be explained by several factors: prolongation of cancer-specific survival even in the metastatic setting in parallel with a poor blood-brain barrier penetration by the newer antineoplastic and targeted therapies, spillage of cancer cells during intracranial surgeries and increased appreciation and diagnosis of the LMC by more accurate modalities (14). The incidence rates of LMC largely depend on tumor type and primary origin (see Table 1). Among solid tumors, it is usually encountered in widespread breast cancer, lung cancer, melanoma and primary brain tumors (14,17,18). A rapidly progressive sensorineural hearing loss is a very rare intriguing manifestation of LMC especially when it occurs as an isolated first presentation (19).

Full table
LMC of gastrointestinal origin is seldom identified. It is reported in up to 14% of patients with primary tumors, commonly originating from the stomach especially of signet ring cell origin, and less commonly from the rectum, colon, gallbladder and pancreas (5,20). In the setting of CRC, the signet ring adenocarcinoma (SRA) appears to be an infrequent histological subtype with an estimated incidence of 0.6 per 100,000 cases per year (21). This entity typically presents at an advanced stage and portends a dismal prognosis (22). The incidence of LMC in CRC has not been clearly established. According to one report by Giglio et al. (20), primary colorectal cancer accounts for only 0.56% of all cases of LMC and the latter complicates CRC in around 0.019% of cases. The rarity of this presentation makes data about natural history, mechanisms of spread and appropriate management sparse and limited to case reports.
This paper will thoroughly address the extremely rare combination of a signet ring cell adenocarcinoma of the colon spreading uniquely to the leptomeninges and manifesting as an isolated bilateral sensorineural deafness.
Clinical presentation
The leptomeningeal spread, being typically disseminated or multifocal, can involve any level of the neuraxis, from cranial nerves to spinal cord and cerebral hemispheres, leading to a wide variety of clinical signs and symptoms (23-26). However, these manifestations are not pathognomonic of LMC as the latter might masquerade like toxicities of antineoplastic agents, encephalopathy, infectious meningitis and multiple parenchymal metastases of the central or peripheral nervous systems (10).
The most frequently affected cranial nerves are III, V, VI, VII, VIII (15,27,28) with diplopia and facial weakness being predominant symptoms. Other alarming manifestations include: headache especially in early morning or induced by posture with absence of parenchymal metastases on imaging studies; ataxia, altered mental status, nausea and vomiting, and spinal symptoms [weakness, paresthesia, neck pain, back pain, radicular pain, bladder and bowel dysfunction with urinary retention being a very early sign of LMC (9,15,29)]. Confusion, memory disturbances and variable cognitive defects may also be evident.
These ambiguous signs and symptoms can sometimes be isolated and precede the discovery of an occult malignancy, prompting a high level of clinical vigilance to establish such a challenging diagnosis.
We report a rare case of LMC as the initial manifestation of recurrent colorectal signet ring carcinoma. An extensive search of the PubMed and Google scholar has yielded 17 cases of LMC arising from CRC that have been previously and fully published in the literature. Including this report, 8 of 18 patients (44%) had CRC with signet ring histology (see Table 2), and 15 (83%) had metastatic disease in sites other than the meninges. In addition, 6 (33%) had LMC as the initial manifestation of their occult malignancy, 12 (66%) as first and isolated site of metastatic CRC, and 10 (55.5%) had hearing loss among the first signs. Interestingly, the hearing defect was never isolated but always accompanied by variable signs and symptoms, including headaches, ataxia, facial paralysis and visual disturbances.
Full table
Our case was unique by the fact that sensorineural hearing loss was isolated, bilateral and of sudden onset, which favored a causative neoplastic process over more benign entities including presbyacusis, ototoxic drugs such as aminoglycosides and diuretics, infections, environmental noise exposure, bilateral acoustic neuroma and Meniere’s disease.
Pathogenic mechanisms
Mechanisms of LMC
According to published literature, the mechanisms by which metastases to leptomeninges occur remain unclear. Four hypotheses have been postulated as to routes of spread:
The theory of hematological spread might largely apply to our case. We think that some colonic malignant clone possessing a yet unknown biological spectrum has survived treatment by surgery and systemic chemotherapy then successfully seeded and implanted into the leptomeningeal spaces making it a sanctuary site of homing.
- Hematological pathway. The communication between the portal system and paravertebral veins allows for metastases to the central nervous system (CNS). In addition, the arterial circulation reaches directly to the arachnoid, and this appears to be the most common route of metastasis, especially in hematological malignancies. Finally, the valveless venous plexus of Batson’s may permit retrograde seeding of the leptomeninges by pelvic tumors (46).
- Direct centripetal spread along peripheral or cranial nerves to the subarachnoid space using the endoneural or perineural or lymphatic trajectories of the nerve roots (47).
- Skull bone marrow micrometastases resulting in perivenous spread into meningeal spaces with efficient malignant cells homing.
- Direct extension from subdural or extradural tumors or from sites outside but adjacent to the CNS (16).
Mechanisms of neural damage
Neoplastic cells may seed into the subarachnoid space causing neurologic damage by several routes (23,48,49). Most often, these cells directly invade the brain, spinal cord, or cranial nerves and spinal roots as they circulate through the subarachnoid space, interfering with the nervous system functions. Models of B16 melanoma clones programmed to metastasize to mice meninges supported this theory by showing that malignant cells first attach to the walls of leptomeningeal capillaries and then move into the subarachnoid space (50). This infiltration might also interrupt absorption of CSF, resulting in manifestations of communicating hydrocephalus. In addition, the perivascular Virchow-Robin spaces might be blocked by tumor deposits, leading to reduced blood supply to the brain, and consequent cerebral infarctions (51).
Finally, major disturbances of the neural metabolism may also occur. This was demonstrated by an experimental model where regional changes in cerebral glucose use have been quantified following LMC (52).
Mechanisms of hearing loss
LMC carcinomatosis presenting with isolated bilateral hearing loss is a very rare occurrence. In fact, the first case of isolated vestibulocochlear nerve (VIII) involvement was reported by Saenger in 1900 (53). A thorough literature review discloses few reports of small series (54,55) and according to Alberts and Terrence (28), this cranial nerve is solely affected by in up to 10% of patients diagnosed with LMC at autopsy. For Wasserstrom et al. (4), some degree of hearing loss was reported in 7 out of 90 reviewed cases of LMC from solid tumors (8%).
The proven mechanisms underlying the hearing loss are still largely unknown but speculated to include the following (Figure 4):
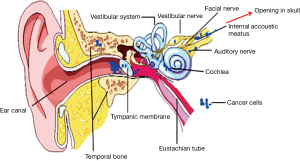
- Tumor cells gain into the internal auditory canal and directly invade the cochlear nerve causing axonal destruction (54,56);
- Malignant cells reach the modiolus through invasion of the cribrose area, directly increasing cochlear dysfunction (55);
- Excessive packing of the internal auditory canal by tumor cells causes vascular compromise by decreasing blood supply from the internal auditory artery to the cochlea, indirectly causing cochlear dysfunction.
Imamura et al. (55) reviewed 10 cases of LMC with bilateral deafness. He found that, in the majority of cases, as in our report, hearing deficits preceded the development of facial paralysis. This may stem from the fact that differences in vulnerability to neoplastic seeding clearly exist between the facial nerve (motor branch) and vestibulocochlear nerve (sensory branch). In addition, most of these patients exhibited either initial bilateral hearing loss or rapidly progressed from unilateral to bilateral deafness. It is therefore hypothesized that malignant cells circulating from CSF concurrently invade the internal auditory canals of both ears, thus leading to bilateral hearing deficits. This simultaneous infiltration and destruction of both vestibular nerves might also result in vertigo and ataxia as noticed in most patients upon presentation or progression of LMC.
Biological aspects
Based on the above speculative mechanisms of spread, the LMC can be seen as a phenomenon controlled by intrinsic factors related to the biology of the primary tumor itself and its properties of distant seeding and homing. Therefore, it does not appear to be a random and chaotic metastatic event especially when it occurs as a primary manifestation of an occult malignancy. For example, the lobular subtype of infiltrating breast cancer often causes LMC, whereas the ductal carcinoma often metastasizes to the brain. In addition, some specific chromosomal abnormalities and eosinophilia in acute myelogenous leukemia (AML) appear to predispose to meningeal chloromas. In support of the theory is a list of molecular markers have been reported to be associated with CNS and leptomeningeal metastases, including, E-cadherin—catenin complexes, plasmin, urokinase type plasminogen activator, metalloproteinases, tissue inhibitors of metalloproteinases (associated with brain invasion) (19), and activated integrin alpha vs. beta-3 (57).
Signet ring cells are a group of malignant cells that have naturally lost the cell-cell adhesion properties, consequently presenting as individual cells or loose clusters (58). This results in diffuse infiltration of the stroma and a propensity to vascular invasion, lymph node involvement and probably distant widespread metastasis.
Regarding CRC, it is well established that high frequency of microsatellite instability portends a favorable prognosis even in mucinous subtypes (59). This, however, contrasts with the fact that this same category of tumors often shows signet-ring morphology, a proven poor prognosticator. Some adverse genetic mutations, including K-ras and p53, were also associated with SRA (60).
In this thorough review we are presenting, we cannot unfortunately correlate the presence of colorectal SRA to a trend toward development of LMC as only 8 out of 18 reported patients (44%) had signet ring cells. We also found no record of molecular studies mainly due to the very short lifespan of patients and the fact that this type of metastatic involvement is quite rare, especially as a first and isolated presentation of an occult or metastatic CRC.
Diagnosis
The diagnosis of LMC can be challenging. It usually relies, as in our case, on abnormalities detected by clinical signs and symptoms, brain MRI and/or CSF cytology.
The identification of neoplastic cells in the CSF establishes the diagnosis of LMC. However, gadolinium-enhanced MRI (6) should be obtained prior to lumbar puncture and should include the brain and the spine as the entire neuraxis might be involved with malignant spread (15). Indeed, MRI has proven to be twice as sensitive and specific as CT scan. Findings suggestive of LMC include meningeal enhancement, the presence of communicating hydrocephalus, and the detection of tumor nodules along the spinal roots even in the absence of overtly symptomatic nerve root dysfunction (9,15,61,62). Finally, brain MRI is precisely sensitive for detecting abnormalities of the internal auditory canals, as is the case with our patient, and may also unveil other possible causes of sensorineural deafness, such as acoustic neuromas.
Despite being the imaging modality of choice, gadolinium-enhanced MRI has high false negative rates (6,14,63,64) and is only 76% sensitive and 77% specific for the detection of LMC (62,64).
When a lumbar puncture is performed, opening pressure measurement should be obtained as well as CSF cell count, protein, glucose, and bacterial and fungal studies to rule out other entities in the differential diagnoses. Abnormal CSF findings include low glucose levels, pleocytosis and elevated protein levels with increased opening pressure (9,14,25).
Assessment of tumor specific markers can also be performed (5,15,25,30,46). When blood-brain barrier is intact, the level of these markers, such as CEA in our case, should not be greater than 1% of the serum level (18). When this ratio is exceeded, especially with a CSF level higher than that of the serum, the diagnosis of LMC is virtually confirmed even in the absence of a positive cytology (65). These markers can be also used for evaluation of disease status and response to treatment.
CSF cytology
The gold standard diagnostic test for LMC is cytology from the CSF or ideally a meningeal biopsy (62). This method was largely abandoned since CSF studies and imaging techniques have emerged as safer and generally accurate diagnostics. A positive CSF cytology is 100% specific for confirmation of LMC; its sensitivity, however, approaches 75% (62). Malignant cells can be missed in the first sample in 40-50% of cases (13,25) prompting additional CSF sampling in an attempt to increase the positivity to 80% after the third trial (13,63,66-69). Therefore, a first negative cytology does not exclude the diagnosis of LMC (9,63,69). Moreover, some authors believe that characteristic findings on MRI are sufficient to establish a diagnosis and eliminate the need for a positive cytology (13,70-72).
In our case, the lumbar puncture revealed the classical findings of positive CEA, raised protein, low glucose levels and confirmative cytology.
Treatment
When untreated, patients with LMC are condemned to constant progression of neurologic dysfunction and ultimate death within days to weeks. Therefore, rapid diagnosis prompting early initiation of treatment is crucial as the development of fixed neurological deficits might be irreversible (15). Around one-half of patients often obtain some improvement of their symptoms with institution of therapy (73).
There is no consensus regarding LMC treatment due to the low number of randomized clinical trials conducted (15). However, treatment usually comprises site-specific radiation therapy in addition to intrathecal and systemic chemotherapy (5,13). Moreover, a ventriculoperitoneal shunt should be considered in the setting of symptomatic hydrocephalus (74). Nevertheless, in view of the dismal prognosis of LMC, the treatment is palliative rather than curative and multiple modalities are often needed to achieve optimal yet unsatisfactory results.
Radiotherapy (RT)
RT is indicated in patients with symptomatic and/or radiological bulky disease (75,76), concomitant brain metastasis and hydrocephalus (15). Starting RT within a maximum of 3 days of symptoms appear to produce more rapid improvement than later treatment, as indicated by studies of LMC in the setting of hematologic malignancies (77). The cranial RT ports must involve the cribriform plate and the base of the skull. The patients often benefit from concomitant dexamethasone in an attempt to improve quality of life, by alleviating nausea, vomiting, and headaches.
Chemotherapy
While systemic chemotherapy aims at controlling the active systemic malignancy (5,13), intrathecal agents [either via lumbar puncture or via an intraventricular (Ommaya) reservoir] bypass the blood-brain barrier, achieve a prolonged exposure and a higher concentration with smaller doses and reduce systemic toxicity (13). However, this can be of limited efficacy in bulky diseases (more than 2 mm in diameter), in tumors in the Virchow-Robin space and along nerve roots (13), in concomitant presence of parenchymal deposits, and in the presence of complete or partial obstruction to CSF flow (78). The use of intrathecal chemotherapy combination has increased toxicity with no superiority over the use of a single agent (13). Because LMC disrupts the blood-brain barrier, systemic chemotherapy, particularly when administered in high doses, may also be effective (63,79).
The choice of agents to be used in intra-CSF largely depends on the tumor type and histology. In this setting, conventional antineoplastic agents usually include methotrexate, cytarabine and thiotepa (5,13). Nevertheless, with regard to LMC in solid tumors, none of these agents has proven clearly superior over any other in the few small randomized studies that have been conducted to date (80-83).
Systemic chemotherapy
The efficacy of systemic chemotherapy in the treatment of LMC is halted by the poor penetration of most agents into the CNS and by the degree of chemoresistance of the underlying primary malignancy. In addition, the choice of agent must also depend on the tumor’s histology as well as prior drug exposure. Although neither drug sufficiently crosses the blood-brain barrier, high doses of intravenous methotrexate (3-8 g/m2) (84,85) or cytarabine (3 g/m2) may generate serum levels high enough to ensure therapeutic levels in the CSF. Newer drugs, such as capecitabine (86), seem to cross the blood-brain barrier more effectively by their intrinsic properties and have been reported to produce potential benefit in the treatment of LMC.
Moreover, in the setting of CRC, Ku et al. (87) reported the unique case of a 49-year-old man with recurrent diffusely metastatic rectal cancer and LMC of the brain and the spinal cord. This patient received a combination of bevacizumab, temozolomide and irinotecan following whole-brain radiation therapy. After four cycles, he had clinical and radiological improvement of the leptomeningeal deposits and systemic disease as well as stabilization of the brain metastasis. He received this treatment for more than 6 months until progression to extensive LMC and finally died of respiratory arrest following aspiration.
Prognostication and outcomes
Unfortunately, LMC still entail a grim prognosis with a median survival of 4-6 weeks if left untreated (5). Patients with signet ring features have more dismal outcomes. At best, the treatment helps controlling the neurological symptoms, slows the progression of the disease and prolongs median survival by only 3-6 months (13,15,88). Despite advances in detection of LMC and in the understanding of its pathophysiology, the response to treatment is still unpredictable and prognostication is deemed difficult. Nevertheless, several models of poor prognostic factors have been developed to assist in decision-making; these include: aggressive underlying malignancy, poor Karnofsky performance status (<60%), extensive CNS metastases, persisting CSF flow bock despite RT, LMC-related encephalopathy, high CSF protein, low glucose and cranial nerve palsy (13).
The histology of underlying primary cancer is the best predictor of response to treatment. For example, most lymphoma and breast cancers are usually relatively sensitive to RT and may occasionally behave as indolent diseases in the leptomeninges. However, providing treatment to patients with severe neurologic dysfunction appears worthless as neural damage is often irreversible.
There exist no standard criteria to evaluate the response to treatment. However, the conversion of the CSF cytology from positive to negative and the improvement or stabilization of the clinical status have been widely used in several clinical trials (13). Recovery or improvement of the hearing loss with treatment has not been reported. In conclusion, LMC should be considered in the differential diagnosis in cases of abrupt onset sensorineural hearing loss. This applies even when there is no known primary malignancy.
Conclusions
As newer therapeutic options prolong patient survival by ensuring long-term control of the systemic manifestations of primary malignancies, the incidence of unusual metastatic sites is expected to rise. This largely applies to LMC of CRC origin where the poor penetrance of the blood-brain barrier by most anticancer agents contributes to designation of leptomeninges as a sanctuary site for malignant cells.
LMC remains a challenging phenomenon with a complex manifold management currently including intra-CSF chemotherapy, RT and several combinations of systemic agents. Unfortunately, outcomes are disappointing with limited benefit in overall survival and symptomatic improvement.
Joint efforts are urgently required for further in-depth understanding of the homing of cancer cells, especially to the CNS, and its molecular basis. Future research and prospective clinical trials should subsequently aim at developing more effective anticancer agents with long half-lives and improved delivery to the CNS and CSF. Treating physicians should also be more vigilant of the risk of this rare type of spread from colorectal adenocarcinomas in order to establish rapid diagnosis and initiate early treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yap HY, Yap BS, Tashima CK, et al. Meningeal carcinomatosis in breast cancer. Cancer 1978;42:283-6. [PubMed]
- Aroney RS, Dalley DN, Chan WK, et al. Meningeal carcinomatosis in small cell carcinoma of the lung. Am J Med 1981;71:26-32. [PubMed]
- Amer MH, Al-Sarraf M, Baker LH, et al. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer 1978;42:660-8. [PubMed]
- Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 1982;49:759-72. [PubMed]
- Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev 1999;25:103-19. [PubMed]
- Chamberlain MC. Neoplastic meningitis: a guide to diagnosis and treatment. Curr Opin Neurol 2000;13:641-8. [PubMed]
- Kato H, Emura S, Takashima T, et al. Gadolinium-enhanced magnetic resonance imaging of meningeal carcinomatosis in colon cancer. Tohoku J Exp Med 1995;176:121-6. [PubMed]
- Eberth CJ. Zur entwicklung des epitheliomas (cholesteatomas) dur pia und der lunge. Virchow’s Arch 1870;49:51-63.
- Kountourakis P, Ardavanis A. Visual and hearing loss due to colorectal meningeal carcinomatosis: a case-based review. Clin Adv Hematol Oncol 2010;8:567-8. [PubMed]
- Kato Y, Takeda H, Dembo T, et al. Progressive multiple cranial nerve palsies as the presenting symptom of meningeal carcinomatosis from occult colon adenocarcinoma. Intern Med 2012;51:795-7. [PubMed]
- Alden TD, Gianino JW, Saclarides TJ. Brain metastases from colorectal cancer. Dis Colon Rectum 1996;39:541-5. [PubMed]
- Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol 1978;19:579-92. [PubMed]
- Glass JP, Melamed M, Chernik NL, et al. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology 1979;29:1369-75. [PubMed]
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg Neurol Int 2013;4:S265-88. [PubMed]
- Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol 2006;5:443-52. [PubMed]
- Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol 1996;53:626-32. [PubMed]
- Marchese MR, La Greca C, Conti G, et al. Sudden onset sensorineural hearing loss caused by meningeal carcinomatosis secondary to occult malignancy: report of two cases. Auris Nasus Larynx 2010;37:515-8. [PubMed]
- Clarke JL. Leptomeningeal metastasis from systemic cancer. Continuum (Minneap Minn) 2012;18:328-42. [PubMed]
- Groves MD, editor. Leptomeningeal Metastases: Still a Challenge. 44th. Chicago, IL: American Society of Clinical Oncology, 2008:80-8.
- Giglio P, Weinberg JS, Forman AD, et al. Neoplastic meningitis in patients with adenocarcinoma of the gastrointestinal tract. Cancer 2005;103:2355-62. [PubMed]
- Kang H, O'Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005;48:1161-8. [PubMed]
- Nissan A, Guillem JG, Paty PB, et al. Signet-ring cell carcinoma of the colon and rectum: a matched control study. Dis Colon Rectum 1999;42:1176-80. [PubMed]
- Posner JB. Neurologic complications of systemic cancer. Dis Mon 1978;25:1-60. [PubMed]
- Aparicio A, Chamberlain MC. Neoplastic meningitis. Curr Neurol Neurosci Rep 2002;2:225-35. [PubMed]
- van Oostenbrugge RJ, Twijnstra A. Presenting features and value of diagnostic procedures in leptomeningeal metastases. Neurology 1999;53:382-5. [PubMed]
- Wolfgang G, Marcus D, Ulrike S. LC. clinical syndrome in different primaries. J Neurooncol 1998;38:103-10. [PubMed]
- Morgan MK, Zammit-Maempel I, Hill J. Meningeal carcinomatosis: an unusual cause of deafness. J R Coll Surg Edinb 1998;43:119-21. [PubMed]
- Alberts MC, Terrence CF. Hearing loss in carcinomatous meningitis. J Laryngol Otol 1978;92:233-41. [PubMed]
- Wang VS, Tuch P, Wang CC. An unusual case of headache. J Clin Neurosci 2003;10:612-6. [PubMed]
- Bresalier RS, Karlin DA. Meningeal metastasis from rectal carcinoma with elevated cerebrospinal fluid carcinoembryonic antigen. Dis Colon Rectum 1979;22:216-7. [PubMed]
- McFadzean R, Brosnahan D, Doyle D, et al. A diagnostic quartet in leptomeningeal infiltration of the optic nerve sheath. J Neuroophthalmol 1994;14:175-82. [PubMed]
- Nagashima T, Muroi K, Kunitama M, et al. Colon cancer with meningeal carcinomatosis and myelodysplastic syndrome in a patient who underwent intensive chemotherapy for acute myelogenous leukemia: a case report. Jpn J Clin Oncol 2001;31:221-5. [PubMed]
- Sartore-Bianchi A, Pedrazzoli P, Ponchio L, et al. Meningeal carcinomatosis in rectal cancer. Clin Oncol (R Coll Radiol) 2002;14:82. [PubMed]
- Jariengprasert C, Laothamatas J, Janwityanujit T, et al. Bilateral sudden sensorineural hearing loss as a presentation of metastatic adenocarcinoma of unknown primary mimicking cerebellopontine angle tumor on the magnetic resonance image. Am J Otolaryngol 2006;27:143-5. [PubMed]
- Longo R, Morabito A, Carillio G, et al. Multiorganic dissemination of a colorectal signet ring cell carcinoma with fulminant clinical course. Int J Gastrointest Cancer 2006;37:49-54. [PubMed]
- Suzuki T, Sakaguchi H, Yamamoto S, et al. Sudden hearing loss due to meningeal carcinomatosis from rectal carcinoma. Auris Nasus Larynx 2006;33:315-9. [PubMed]
- Chadha MK, Iyer R, Nava ME, et al. Leptomeningeal metastases from signet ring adenocarcinoma of the cecum. J Clin Oncol 2007;25:5028-9. [PubMed]
- Crncevic-Urek M, Bokun T, Kujundzic M, et al. Leptomeninges as the first and only dissemination site of colorectal cancer. Int J Colorectal Dis 2009;24:355-6. [PubMed]
- Su PJ, Hsieh CH, Yang TS. Leptomeningeal Carcinomatosis from a Primary Colon Cancer Patient. J Cancer Res Pract 2011;27:113-6.
- Bruce BB, Tehrani M, Newman NJ, et al. Deafness and blindness as a presentation of colorectal meningeal carcinomatosis. Clin Adv Hematol Oncol 2010;8:564-6. [PubMed]
- Yildirim Y, Akcali Z, Ozyilkan O. Signet-ring cell carcinoma of the colon with leptomeningeal involvement. J Clin Neurosci 2010;17:1051-3. [PubMed]
- Lahiri RP, Burnand KM, Bandi A, et al. Colorectal cancer presenting with dysarthria and ataxia: a case of isolated leptomeningeal metastasis. Ann R Coll Surg Engl 2011;93:e133-5. [PubMed]
- Kleinfeld K, Peters WH, Stephenson C, et al. Leptomeningeal metastasis from occult signet-ring cell colon adenocarcinoma presenting with isolated headache. J Clin Neurosci 2013;20:890-2. [PubMed]
- Mohebi N, Moghaddasi M, Karimian H. Diffuse leptomeningeal metastasis from signet-ring cell adenocarcinoma of the sigmoid. Journal of Case Reports in Practice 2014;2:34-6. (JCRP).
- Traşcă D, Şerban AS, Ştefănescu V, et al. Meningeal carcinomatosis in a patient with Crohn's disease. Rom J Intern Med 2014;52:111-20. [PubMed]
- Clouston PD, DeAngelis LM, Posner JB. The spectrum of neurological disease in patients with systemic cancer. Ann Neurol 1992;31:268-73. [PubMed]
- Olson ME, Chernik NL, Posner JB. Infiltration of the leptomeninges by systemic cancer. A clinical and pathologic study. Arch Neurol 1974;30:122-37. [PubMed]
- Mareel M, Leroy A, Bracke M. Cellular and molecular mechanisms of metastasis as applied to carcinomatous meningitis. J Neurooncol 1998;38:97-102. [PubMed]
- Kastenbauer S, Wiesmann M, Pfister HW. Cerebral vasculopathy and multiple infarctions in a woman with carcinomatous meningitis while on treatment with intrathecal methotrexate. J Neurooncol 2000;48:41-5. [PubMed]
- Nicolson GL, Kawaguchi T, Kawaguchi M, et al. Brain surface invasion and metastasis of murine malignant melanoma variants. J Neurooncol 1987;4:209-18. [PubMed]
- Klein P, Haley EC, Wooten GF, et al. Focal cerebral infarctions associated with perivascular tumor infiltrates in carcinomatous leptomeningeal metastases. Arch Neurol 1989;46:1149-52. [PubMed]
- Hiesiger EM, Picco-Del Bo A, Lipschutz LE, et al. Experimental meningeal carcinomatosis selectively depresses local cerebral glucose utilization in rat brain. Neurology 1989;39:90-5. [PubMed]
- Saenger A. Uber hirnsymptome bei carcinomatose. Munch Med Wochenschr 1900;47:341-2.
- Civantos F, Choi YS, Applebaum EL. Meningeal carcinomatosis producing bilateral sudden hearing loss: a case report. Am J Otol 1992;13:369-71. [PubMed]
- Imamura S, Nozawa I, Imamura M, et al. Clinicopathologic study of leptomeningeal carcinomatosis involving the temporal bone. Ann Otol Rhinol Laryngol 1997;106:674-9. [PubMed]
- Oshiro H, Perlman HB. Subarachnoid spread of tumor in the labyrinth. Arch Otolaryngol 1965;81:328-34.
- Lorger M, Krueger JS, O'Neal M, et al. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A 2009;106:10666-71. [PubMed]
- Börger ME, Gosens MJ, Jeuken JW, et al. Signet ring cell differentiation in mucinous colorectal carcinoma. J Pathol 2007;212:278-86. [PubMed]
- Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev 2001;10:917-23. [PubMed]
- Groves MD. New strategies in the management of leptomeningeal metastases. Arch Neurol 2010;67:305-12. [PubMed]
- Chamberlain MC. Comparative spine imaging in leptomeningeal metastases. J Neurooncol 1995;23:233-8. [PubMed]
- Straathof CS, de Bruin HG, Dippel DW, et al. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol 1999;246:810-4. [PubMed]
- Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 1998;82:733-9. [PubMed]
- von Campe G, Regli F, Confavreux C, et al. Bilateral deafness, an initial manifestation of meningeal carcinomatosis. Rev Neurol (Paris) 1992;148:133-8. [PubMed]
- Kobayashi TK, Yamaki T, Yoshino E, et al. Immunocytochemical demonstration of carcinoembryonic antigen in cerebrospinal fluid with carcinomatous meningitis from rectal cancer. Acta Cytol 1984;28:430-4. [PubMed]
- Chamberlain MC. Leptomeningeal metastases: a review of evaluation and treatment. J Neurooncol 1998;37:271-84. [PubMed]
- Chamberlain MC, Glantz M, Groves MD, et al. Diagnostic tools for neoplastic meningitis: detecting disease, identifying patient risk, and determining benefit of treatment. Semin Oncol 2009;36:S35-45. [PubMed]
- Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol 2010;22:627-35. [PubMed]
- Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol 2004;15:iv285-91. [PubMed]
- Schumacher M, Orszagh M. Imaging techniques in neoplastic meningiosis. J Neurooncol 1998;38:111-20. [PubMed]
- Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol 1995;38:51-7. [PubMed]
- Collie DA, Brush JP, Lammie GA, et al. Imaging features of leptomeningeal metastases. Clin Radiol 1999;54:765-71. [PubMed]
- Hildebrand J. Prophylaxis and treatment of leptomeningeal carcinomatosis in solid tumors of adulthood. J Neurooncol 1998;38:193-8. [PubMed]
- Omuro AM, Lallana EC, Bilsky MH, et al. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology 2005;64:1625-7. [PubMed]
- Hanssens PE, Lagerwaard FJ, Levendag PC. Principles of radiotherapy of neoplastic meningosis. J Neurooncol 1998;38:145-50. [PubMed]
- Chang EL, Maor MH. Standard and novel radiotherapeutic approaches to neoplastic meningitis. Curr Oncol Rep 2003;5:24-8. [PubMed]
- Gray JR, Wallner KE. Reversal of cranial nerve dysfunction with radiation therapy in adults with lymphoma and leukemia. Int J Radiat Oncol Biol Phys 1990;19:439-44. [PubMed]
- Grossman SA, Trump DL, Chen DC, et al. Cerebrospinal fluid flow abnormalities in patients with neoplastic meningitis. An evaluation using 111indium-DTPA ventriculography. Am J Med 1982;73:641-7. [PubMed]
- Siegal T. Leptomeningeal metastases: rationale for systemic chemotherapy or what is the role of intra-CSF-chemotherapy? J Neurooncol 1998;38:151-7. [PubMed]
- Hitchins RN, Bell DR, Woods RL, et al. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol 1987;5:1655-62. [PubMed]
- Grossman SA, Finkelstein DM, Ruckdeschel JC, et al. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol 1993;11:561-9. [PubMed]
- Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res 1999;5:3394-402. [PubMed]
- Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer 2004;40:2726-33. [PubMed]
- Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol 1998;16:1561-7. [PubMed]
- Lassman AB, Abrey LE, Shah GD, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol 2006;78:255-60. [PubMed]
- Tham YL, Hinckley L, Teh BS, et al. Long-term clinical response in leptomeningeal metastases from breast cancer treated with capecitabine monotherapy: a case report. Clin Breast Cancer 2006;7:164-6. [PubMed]
- Ku GY, Krol G, Ilson DH. Successful treatment of leptomeningeal disease in colorectal cancer with a regimen of bevacizumab, temozolomide, and irinotecan. J Clin Oncol 2007;25:e14-6. [PubMed]
- Nakagawa H, Murasawa A, Kubo S, et al. Diagnosis and treatment of patients with meningeal carcinomatosis. J Neurooncol 1992;13:81-9. [PubMed]


