Effectiveness of early and aggressive administration of fresh frozen plasma to reduce massive blood transfusion during cytoreductive surgery
Introduction
The perception that peritoneal carcinomatosis (PC) is invariably fatal continues to be challenged. Over the last 14 years, several phase II studies have demonstrated improved survival in selected patients treated with cytoreductive surgery (CRS) and perioperative intraperitoneal chemotherapy (PIC) (1,2). Moreover, a single randomized trial performed by the Netherlands Cancer Institute demonstrated the superiority of CRS and hyperthermic intraperitoneal chemotherapy (HIPEC) compared to palliative therapy in patients with isolated colorectal peritoneal carcinomatosis (3).
Unfortunately, due to the complexity of the surgical techniques, these procedures are often accompanied by substantial intraoperative blood loss and hence require red blood cell (RBC) transfusion. High rates of RBC transfusion ranging from 40-80% have been reported for peritonectomy procedures and a significant proportion of these patients required massive blood transfusion of more than 5 units (3-6). A previous study by our institution showed that 37% of patients required transfusion of ≥6 units of RBC (7). This has significant clinical implications. Blood transfusion is a costly product associated with significant infectious and non-infectious risks (8). It may also down-regulate immune function. The key implication of this is an increased risk of postoperative infectious complications and earlier tumour recurrence. This has been extensively reported in surgical oncology (9,10). And since peritonectomy patients are often massively transfused, the risks are particularly substantial.
At our institution, patients with a significant risk of intraoperative massive blood transfusion because of high volume disease (PCI ≥16) were selected for a new approach. This involved the early and aggressive administration of fresh frozen plasma (FFP) and restriction of crystalloid based resuscitation. Our strategy contrasts with the standard approach to resuscitation which emphasizes the use of crystalloids and RBCs to improve cardiac output and oxygen delivery whilst restricting the use of procoagulant factors. This study evaluates the impact of introducing this protocol on the timing of blood component transfusion and its effectiveness in reducing overall intraoperative transfusion over a period of 13 years. The implications of this study are significant and extend beyond the specialized field of cytoreductive surgery.
Methods
Selection criteria
The current study was approved by an institutional review board and ethics committee. Informed consent was obtained from all patients regarding access to their medical records. This study analysed 131 consecutive patients with high volume disease who underwent CRS combined with PIC between February 1996 and January 2009. High volume disease was arbitrarily defined as PCI ≥16. We have previously shown a significantly increased risk of massive blood transfusion in patients with a PCI ≥16 (6). Patients were deemed suitable for CRS and PIC through consensus of a multidisciplinary team. All patients had biopsy confirmed diagnosis of peritoneal carcinomatosis. Preoperative investigations performed to aid disease assessment included history, physical examination, tumour markers and contrast enhanced abdominal, pelvic and chest CT. Positron emission tomography (PET) was performed in recent years for patients with a diagnosis of colorectal peritoneal carcinomatosis and selectively in other high-grade disease types.
CRS and PIC was offered to patients who were <80 years old, with a good performance status (World Health Performance Status ≤2), and adequate hematological, hepatic, cardiac and liver function. Patients with extra-abdominal metastasis were excluded. Patients were admitted day before surgery. On admission, 5,000 units of subcutaneous heparin were administered twice a day to all patients. The anaesthesia risk was assessed by using the American Society of Anaesthesiologists (ASA) classification (11).
Cytoreductive surgery
All cytoreductive procedures were performed by a single surgeon (D.L.M.). The volume and extent of the tumour deposits were recorded using the Peritoneal Cancer Index (PCI) proposed by Sugarbaker (7). Peritonectomy procedures were then performed according to Sugarbaker’s guidelines (12). These included total anterior parietal peritonectomy, omentectomy ± splenectomy, right and left upper quadrant peritonectomy, pelvic peritonectomy and lesser omentectomy ± cholecystectomy. Omentectomy was performed where indicated. Commensurate with the findings of other studies it was performed in the majority of, but not all, patients (13). The standard dissection tool was the 0.3 mm ball-tip diathermy. This minimised blood loss from small vessels up to 1.5 mm in diameter. Larger vessels were electro-coagulated or ligated in continuity and divided.
Visceral resections were performed at anatomic sites where tumour deposits were infiltrating deeply into an organ rendering surface excision ineffectual. The aim of CRS was to achieve no visible disease. Following the surgical procedures all sites and volumes of residual disease were prospectively recorded using the Completeness of Cytoreduction (CCR) Score (11). The abdomen was explored for hemostasis to prevent blood loss during HIPEC or after abdominal closure.
Perioperative intraperitoneal chemotherapy
After the cytoreduction was completed, but before intestinal anastomosis or repair of seromuscular tears, the abdomen and pelvic were instilled with HIPEC in the operating room at approximately 42 oC in 3 L of 1.5% dextrose peritoneal dialysis solution for 90 minutes. The coliseum technique was used. For gastrointestinal malignancies, mitomycin C (10-12.5 mg/m2) was used. For mesothelioma and ovarian malignancies, cisplatin (50 mg/m2) and doxorubicin (15 mg/m2) or mitomycin C (10-12.5 mg/m2) were employed. Early postoperative intraperitoneal chemotherapy was postoperatively administered in accordance with previously defined guidelines (4).
Anaesthesia
At our institution, the indication for intraoperative RBC transfusion was a hemoglobin concentration <80 g/L and/or signs of anaemia (sinus tachycardia with a heart rate of >100 per minute, a systolic blood pressure of <100 mmHg, urine output of <30 mL/hr due to ongoing blood loss). To minimise unnecessary blood loss we have focused on maintaining normothermia and optimimizing acid-base balance. In the operating theatre, forced warm air blankets were applied and the head was covered with a heat reflecting cap. All fluids and blood products were infused via fluid warming devices. Acidosis was managed by resuscitation and mechanical ventilation. Our response to fluid resuscitation and coagulopathy in patients expected to require an extensive procedure because of high volume disease has changed since June, 2006. Previously, we followed the standard resuscitation strategy with an emphasis on crystalloid and RBC administration to improve cardiac output and oxygen delivery. Procoagulant factors (FFP, cryoprecipitate, platelets) were transfused in response to abnormal coagulation laboratory parameters, hypotension unresponsive to crystalloid administration, transfusion >6 units or obvious microvascular bleeding. Since June 2006, a protocol driven approach has been adopted which involves the early and aggressive administration of procoagulant factors (particularly FFP) to prevent rather than treat coagulopathy. In particular it was deemed not necessary to wait for laboratory results before initiating administration of procoagulants. Procoagulant factors were organized to be available from the outset of surgery and were given such that the ratio of FFP:RBC administered exceeded 1:1, even through the early intraoperative period. Moreover, a fluid restriction strategy was pursued to minimize dilutional coagulopathy.
The rationale behind this change in strategy was multifactorial. Firstly, recent data from trauma surgery has shown that current resuscitation strategies severely underestimate the degree of coagulopathy during surgery. Secondly, our own experience showed that waiting for laboratory results before administering procoagulant factors often resulted in marked coagulation defects and significant blood loss, which could be reduced with a pre-emptive strategy. Thirdly, the association of significant blood loss and subsequent transfusion with poorer perioperative and survival outcomes has been extensively documented. Given these factors, this strategy was approved by the relevant committee’s and adopted as protocol by the St George Hospital Department of Anaesthetics.
Study methods
For study purposes, patients treated before and after June, 2006 have been categorized into treatment period I and II respectively. Patient and procedural data was collected and recorded in a prospective database. Anaesthetic variables such as the intraoperative transfusion of blood components (FFP, RBC, cryoprecipitate, platelets, 4% human serum albumin) and fluids (crystalloids, colloids) were prospectively recorded in operative anaesthetic charts. The timing of intraoperative blood component and fluid transfusion was carefully recorded. The ratio of FFP transfused in the first half of the surgical intervention relative to the second half was calculated (FFP1st:FFP2nd ratio). Similarly, the ratio of RBC transfused in the first half of the surgical intervention relative to the second half was calculated (RBC1st:RBC2nd ratio). A consensus on the definition of massive blood transfusion has not been established among all peritonectomy centres. In our institution massive blood transfusion was defined as ≥6 units of RBC transfused intraoperatively. This definition is consistent with a previous study from our institution (6).
The clinical and treatment-related data were compared between the two groups. Categorical variables were compared using the Chi2 analysis or Fisher’s exact test where appropriate. Significance was defined as P<0.05. Statistical analysis was performed using SPSS software (Version 16.0; GmbH, Munich, Germany).
Results
Descriptive data
A total of 131 procedures performed between February 1996 and January 2009 were evaluated. Seventy-one (54%) procedures were performed subsequent to June 2006. The mean age of the study cohort at the time of surgery was 51 (S.D =12) years and 63 (48%) patients were male. The ASA classification was <3 in 49 patients (37%) and ≥3 in 77 patients (59%). In 7 patients (5%) no ASA score was recorded. The primary histological diagnosis included pseudomyxoma peritonei (n=93, 71%), colorectal peritoneal carcinomatosis (n=12, 9%), peritoneal mesothelioma (n=14, 11%) and peritoneal neoplasms of other origins (n=12, 9%). The mean PCI per patient was 24 (S.D =7).
Intraoperatively, the mean operative duration was 11 (S.D =4). In 112 procedures (85%) optimal cytoreduction (CC0) was achieved. In 12 procedures (15%) cytoreduction was suboptimal (CC1/CC2/CC3). The mean number of peritonectomy procedures performed was 4 (S.D =2). Small bowel resection, colonic resection, gastrectomy, hysterectomy/ bilateral salpingo-oophorectomy and hepatectomy were performed in 60 (46%), 90 (73%), 12 (9%), 12 (9%) and 8 (6%) procedures respectively. HIPEC was administered in 118 (90%) procedures. Procedural data is shown in Table 1.
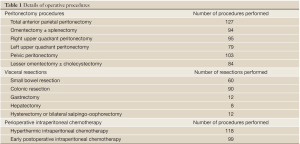
Full table
One-hundred and seventeen procedures (89%) required transfusion of red blood cells. Seventy procedures (53%) required massive red blood cell transfusion (≥6 units). The mean red blood cell transfusion was 8 (S.D =8; median =6; range, 0-38) units. One-hundred and fifteen procedures (88%) required transfusion of fresh frozen plasma (FFP). The mean FFP transfusion was 9.5 (S.D =7; median =8; range, =0-34) units. Other blood products transfused included platelets (mean =1 unit; S.D =3; median =0 unit; range, 0-20 unit), cryoprecipitate (mean =7 units; S.D =9.5; median =0 unit; range, 0-50 unit) and 4% human albumin solution (mean =3.5 L; S.D =3; median = 3.5 L; range, 0-12.5 L).
The mean crystalloid administration was 5 L (S.D =3.5; median = 4 L; range, 0-17 L). The mean colloid administration was 1 L (S.D =2; median = 0 L; range, 0-11.5 L).
Comparison of clinical characteristics between the initial 60 patients (Group I) and subsequent 71 patients (Group II)
Table 2 Demonstrates the comparison of eleven clinical factors between the two treatment groups. There was no significant difference in the clinical characteristics between the two treatment periods
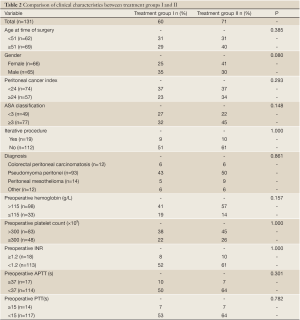
Full table
Comparison of treatment-related factors between the initial 60 patients (Group I) and subsequent 71 patients (Group II)
Table 3 Demonstrates the comparison of twelve treatment-related factors between the two treatment groups. Treatment period II was associated with operation length <11 hours (P<0.001). It was also associated with red blood cell transfusion <6 units (P<0.001), fresh frozen plasma transfusion <10 units (P=0.024), FFP1st:FFP2nd ratio >1 (P<0.001), RBC1st:RBC2nd ratio ≥1 (P=0.016), cryoprecipitate transfusion <10 units (P=0.020), nil platelet transfusion (P<0.001), crystalloid administration <5 L (P<0.001) and colloid administration <1 L (P<0.001). A significantly lower proportion of patients in Group II required RBC transfusion (82% vs. 97%, P=0.009). The mean RBC transfusion was significantly lower in Group II (5.8 vs. 10.9 units, P<0.001).
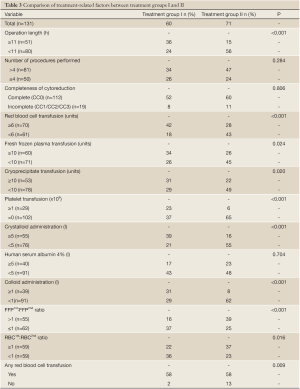
Full table
Comparison of peri-operative outcomes between the initial 60 patients (Group I) and subsequent 71 patients (Group II)
Table 4 Demonstrates the comparison of peri-operative mortality and morbidity between the two treatment groups. Group II patients were more likely to develop ileus post-operatively (P<0.001); conversely, they were less likely to develop perforated viscus (P=0.04). The incidence of other complications was similar in both groups. There was no difference in the incidence of peri-operative mortality between the two groups (P=0.703).
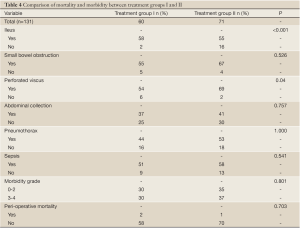
Full table
Discussion
Background
Several phase II studies have demonstrated that CRS and PIC prolongs survival in selected patients with PC (1-3). Unfortunately, CRS is invariably long and patients often lose significant quantities of blood as a result of microvascular bleeding from the extensive raw surfaces that remain after peritoneum stripping (3,5,14). Consequently, transfusion of red blood cells is usually necessary. This has significant clinical implications. Verwaal et al. demonstrated that after massive blood loss (>5 L) the chance of complicated recovery increased sharply to 100% (15). Other studies in CRS identified blood loss as a predictor of extra-abdominal complications (14), and overall complications (3,5). Transfusion of RBC is an expensive solution to intraoperative blood loss, which is associated with substantial risk. These include infectious risks such as HIV, hepatitis B and hepatitis C and non-infectious risks such as hemolytic reactions, acute lung injury and graft versus host disease (7). Most concerning for peritonectomy patients is that transfusion impairs various functions of cellular immunity (16). The key implication of this is an increased risk of postoperative infections and greater cancer recurrence. In 2002, a meta-analysis established association between ABT and postoperative bacterial infections (17). Four years later, a meta-analysis of 36 studies showed a consistently detrimental association between blood transfusion and colorectal cancer recurrence (18).
Outcome of changed anaesthetic approach
In our first ten years of experience with this procedure, 70% of patients with high disease volume required massive red blood cell transfusion as a result of blood loss. We observed that these patients, who were managed by transfusion of RBC and crystalloid, often developed significant blood loss in the latter half of the surgical intervention. Once massive blood loss had occurred (>6 units) or laboratory parameters demonstrated abnormal coagulation, procoagulant factors (FFP, cryoprecipitate, platelets) were aggressively delivered in an ad-hoc manner. A significant amount of time and resources were spent on ensuring hemostasis independent of any surgical procedure. Given the deleterious effects of massive transfusion, we initiated an aggressive anaesthetic program to reduce transfusion in patients with high volume disease in June 2006. The primary intervention was the early and aggressive administration of FFP and restriction of fluid administration to prevent rather than treat coagulopathy and blood loss.
We have since observed a significantly reduced rate of not only massive red blood cell transfusion but also transfusion of other blood products. Moreover, there has been a significant shift in the timing of blood product transfusion. Treatment period II was associated with an increased transfusion of both FFP and RBC during the first half of the surgical intervention relative to the second half (P<0.001). There was a simultaneous decrease in the amount of crystalloid and colloid administered (P<0.001). These data imply that early delivery of procoagulant factors combined with a restrictive fluid approach can prevent the development of progressive systemic coagulopathy and thus reduce transfusion of RBC and procoagulant factors later during the intervention. Most importantly, it can reduce the overall transfusion of all blood products. Our approach conflicts with traditional resuscitation strategies which emphasise increased transfusion of RBC units and crystalloid to maintain blood pressure and oxygen delivery. However, since neither RBCs nor crystalloid contain procoagulant factors this practice dilutes the concentration of clotting factors and impairs fibrinogen polymerisation, therefore contributing to the development of coagulopathy. In contrast FFP contains approximately 400 mg of fibrinogen, the final effector in the clotting system shown to decrease early in patients with haemorrhage (19-21). FFP also has the additional benefit of acting as a buffer, potentially improving the acid base status of patients who are already acidotic. This is in contrast to the use of crystalloids that are acidic in nature and proinflammatory (22-24).
Aggressive anaesthetic strategy in other surgical procedures
Previous studies on liver transplantation and cardiac surgery identified little or no reduction in blood loss with early administration of FFP (25). However, there has been a recent upsurge of interest re-examining the role of FFP in trauma surgery. In 2003, Hirshberg et al. used mathematical modelling to simulate the dilutional component of coagulopathy in haemorrhagic trauma patients. They concluded that existing resuscitation strategies severely underestimated the dilution of coagulation factors and recommended giving FFP concurrently with the first units of blood when the surgeon anticipates severe haemorrhage to prevent the exponential dilution of coagulation factors (26). Several subsequent studies on trauma patients have supported the increased use of plasma early in the course of surgery in patients expected to require massive transfusion (27-31). Though CRS is performed as an elective procedure it is a massive undertaking especially in patients with high volume disease. Patients are exposed to massive fluid shifts, electrolyte imbalances in addition to blood loss. Therefore, in the absence of an aggressive anaesthetic approach coagulopathy is extremely likely to develop.
Limitations of this study
It is possible that the reduced transfusion of all blood products over time reflects a general improvement in surgical technique as part of the “learning curve”, that is improved outcomes secondary to increased familiarisation and experience with surgery. Unfortunately, this is difficult to assess. Another potentially confounding factor is the adoption of new surgical technology over the 13 year study period. This could have reduced bleeding and diluted the observed results. However, the majority of patients in the current study were treated in the last 6 years due to the increased acceptance of CRS in recent years. Furthermore, it must be noted that new surgical technology has not been specifically introduced since the adoption of the novel anaesthetic protocol. In this regard, changes in outcome are more likely due to the anaesthetic intervention which was implemented rather than other factors.
Moreover, this study shows a significant decrease in operation duration, a known risk factor for blood component transfusion over time (P<0.001) (6). However, there has been no significant change in the complexity (number of peritonectomy procedures) of surgery performed. The decreased operative duration is therefore likely to be partly related to the reduced time spent correcting hemodynamic instability late in the surgical intervention. The major shortcoming of this study is its observational design. A randomised controlled trial comparing transfusion outcomes in patients treated with a traditional resuscitation strategy versus our novel anaesthetic approach would provide the strongest evidence. This has not been performed to date. Until then, this study provides evidence on the advantages of an aggressive resuscitation strategy during long and complex surgery.
Conclusions
In conclusion, our study demonstrates that early administration of fresh frozen plasma combined with restrictive fluid resuscitation over traditional resuscitation strategies may reduce overall blood component transfusion. The results of this study have a broader significance than CRS. Given the need to minimise the risks associated with blood component therapy, our results warrant further investigation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yan TD, Black D, Savady R, et al. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 2007;14:484-92.
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681-5.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43.
- Yan TD, Links M, Fransi S, et al. Learning curve for cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal surface malignancy--a journey to becoming a Nationally Funded Peritonectomy Center. Ann Surg Oncol 2007;14:2270-80.
- Hansson J, Graf W, Påhlman L, et al. Postoperative adverse events and long-term survival after cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 2009;35:202-8.
- Saxena A, Yan TD, Chua TC, et al. Risk factors for massive blood transfusion in cytoreductive surgery: a multivariate analysis of 243 procedures. Ann Surg Oncol 2009;16:2195-203.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74.
- Weber RS, Jabbour N, Martin RC 2nd. Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol 2008;15:34-45.
- Hyung WJ, Noh SH, Shin DW, et al. Adverse effects of perioperative transfusion on patients with stage III and IV gastric cancer. Ann Surg Oncol 2002;9:5-12.
- Makino Y, Yamanoi A, Kimoto T, et al. The influence of perioperative blood transfusion on intrahepatic recurrence after curative resection of hepatocellular carcinoma. Am J Gastroenterol 2000;95:1294-300.
- ASA. New classification of physical status. Anesthesiology 1963;24:111.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42.
- Yan TD, Edwards G, Alderman R, et al. Morbidity and mortality assessment of cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma--a prospective study of 70 consecutive cases. Ann Surg Oncol 2007;14:515-25.
- Elias D, Goere D, Blot F, et al. Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: mortality and morbidity in 106 consecutive patients. Ann Surg Oncol 2007;14:1818-24
- Verwaal VJ, van Tinteren H, Ruth SV, et al. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol 2004;85:61-7.
- Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev 2002;16:144-60.
- Hill GE, Frawley WH, Griffith KE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma 2003;54:908-14.
- Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev 2006;(1):CD005033.
- Hess JR. Blood and coagulation support in trauma care. Hematology Am Soc Hematol Educ Program 2007:187-91.
- Fries D, Innerhofer P, Reif C, et al. The effect of fibrinogen substitution on reversal of dilutional coagulopathy: an in vitro model. Anesth Analg 2006;102:347-51.
- Martini WZ, Chinkes DL, Pusateri AE, et al. Acute changes in fibrinogen metabolism and coagulation after hemorrhage in pigs. Am J Physiol Endocrinol Metab 2005;289:E930-4.
- Alam HB, Rhee P. New developments in fluid resuscitation. Surg Clin North Am 2007;87:55-72, vi.
- Cotton BA, Guy JS, Morris JA Jr, et al. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock 2006;26:115-21.
- Kiraly LN, Differding JA, Enomoto TM, et al. Resuscitation with normal saline (NS) vs. lactated ringers (LR) modulates hypercoagulability and leads to increased blood loss in an uncontrolled hemorrhagic shock swine model. J Trauma 2006;61:57-64; discussion 64-5.
- Stanworth SJ, Brunskill SJ, Hyde CJ, et al. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol 2004;126:139-52.
- Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454-63.
- Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg 2008;248:447-58.
- Gunter OL Jr, Au BK, Isbell JM, et al. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma 2008;65:527-34.
- Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma 2008;64:1177-82; discussion 1182-3.
- Cotton BA, Au BK, Nunez TC, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma 2009;66:41-8
- Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma 2007;62:112-9.


