Widespread osteoblastic metastases and marked elevation of CA19-9 as a presentation of signet ring cell gastric carcinoma
Introduction
The potential of cancer to spread to other sites, rather than the actual occurrence of a tumor in a primary site, is what turns it into a fatal disease in most cancer patients (1). Skeletal metastases appear in many cancers but are most commonly observed in patients with breast or prostate cancer. It is presumed that in such patients the bulk of the tumor burden at the time of death would appear in the skeleton (1).
Traditionally, skeletal metastases are classified into two major categories: osteolytic and osteoblastic lesions. Osteolytic lesions, most prominently occurring in breast cancer, are characterized by excessive activation of osteoclasts within the bone. In these cases the dominant process occurring in the bone is lytic, which is destructive by nature. In contrast, in osteoblastic lesions, most prominently occurring in prostate cancer, the characteristic process is excessive activation of osteoblasts adjacent to metastatic tumor cells. This activation may be revealed by an elevation in such biochemical markers as Alkaline Phosphatase (ALP) and Osteocalcin.
It has been demonstrated in recent years, however, that the classification of bone metastases into pure osteolytic or osteoblastic is largely inaccurate (1,2). For example, it is estimated that in 15-20% of patients with bone metastases originating from breast tumors, concurrent osteoblastic activity may be observed, as indicated by increased levels of ALP in the serum, and by bone scanning agents at the skeletal site. Furthermore, the destruction of bone in osteolytic lesions alone is known to induce a secondary osteoblastic effect, resulting in bone formation. Though beyond the scope of this case report, it should be noted that in recent years a vast research endeavor aims to elucidate the mechanisms which underlie the different patterns of bone metastases (3,4).
In the case reported herein, a patient with gastric signet ring cell carcinoma had multiple sclerotic osteoblastic metastases and significant elevation of the tumor marker CA19-9 at presentation. Only very few cases of gastric signet ring cell carcinoma and osteoblastic metastases have been described in the literature, and in one report an increase in CA19-9 was also documented. The precise mechanism which underlies the formation of osteoblastic metastases, as well as the role of CA19-9 in gastric cancer, are not well defined.
Case presentation
A healthy 45-year-old male was admitted to our ward due to having a fever during the previous 10 days accompanied by chest pains during the month prior to hospitalization. Chest pain, appearing only at night, during rest, and lasting for several minutes, was described as pressing, unstable, non-pleuritic, unrelated to meals, without radiations, and with an intensity of 7/10. The fevers, which rose as high as 38 degrees Celsius, were accompanied by fatigue and chills. Additional symptoms in the month prior to admission included left tibial pain which appeared after pressing, stretching or when beginning to walk. Pain intensity, which lasted for only one week, was described as 3/10; right tibial pain appeared following disappearance of the left tibial pain; right cervical pain appeared while stretching; and loss of 2 kg within 1 month occurred despite preserved appetite. The patient negated any gastro-intestinal symptoms.
Physical examination revealed a well-nourished patient with a BMI of 26. Vital signs were normal at admission. A mild non-specific yellowish-pale change in skin color was apparent. There was no palpable lymph node or axial skeleton sensitivity or deformity. Abdominal examination revealed no tenderness or organomegaly.
Laboratory examination at admission revealed the following data: complete blood count within normal limits (WNL) except mild normocytic anemia (13.9 g/dL/90 µm3). serum creatinine, urea, calcium, phosphate, total bilirubin, total protein and albumin were WNL. Liver enzymes were WNL except ALP level—289 IU/L (normal limits: 40-129 IU/L) and LDH 649 U/L (240-480 U/L). CRP level was 31.4 mg/L (0.5-6 mg/L). The serum level of tumor marker CA 19-9 was markedly increased with 8,141 U/mL (0-36 U/mL), other tumor markers were mildly increased—CA15-3 35.5 U/mL (0-28 U/mL), carcino-embryonic antigen (CEA) 17 ng/mL (0-4 ng/mL), NSE 27.8 ng/mL (0-16.3 ng/mL), and α-fetoprotein and PSA were WNL. Results of urinalysis were normal. Chest radiograph and ultrasound of upper GI, kidneys, testicles and thyroid gland yielded no significant findings.
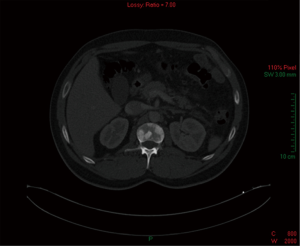
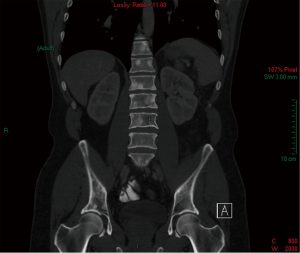
Abdominal, pelvic and chest CT revealed multiple sclerotic skeletal lesions in the vertebrae and pelvic bones (Figures 1-3). Small nodules in the mediastinum and retroperitoneum were observed. No evidence of ileac or pelvic lymphadenopathy was observed. In addition, no evidence of focal findings in the stomach, liver, kidneys, adrenals, pancreas or lungs was observed.
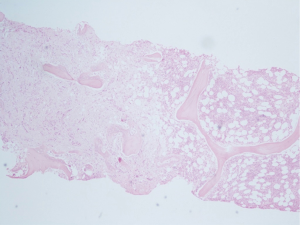
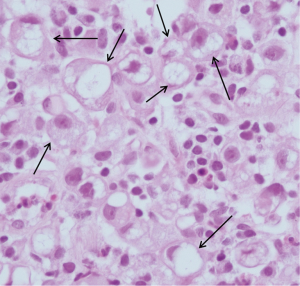
A random pelvic bone marrow biopsy demonstrated poorly differentiated signet ring cells of stomach origin which stained positive for Cytokeratin 7 and negative for Cytokeratin 20 (Figure 4). Gastroscopy showed two pathological regions within the stomach: limited and slightly elevated stiff tissue in the greater curvature, and chronic active gastritis associated with Helicobacter Pylori. Gastric biopsy showed poorly differentiated signet ring cell carcinoma compatible with the skeletal biopsy (Figures 5,6). HER2-neu was negative in tumor cells. Colonoscopy showed no evidence of pathology in the colon.

Discussion
This case report presents a unique example of widespread osteoblastic skeletal metastases and markedly elevated CA19-9 as the initial presentation of signet ring cell gastric carcinoma.
Only a handful of similar cases have been described in the literature, with only one case that indicated additional increase in CA19-9 (5-7). The vast majority of cases with osteoblastic bone metastases in the literature are attributed to prostatic carcinoma (1,4,8), whereas most cases of marked elevations of CA19-9 are associated with pancreatic carcinoma (9-11).
The patient reported a variety of signs and symptoms which at first did not seem to derive from a single unifying source. These symptoms included episodes of chest pain, nocturnal fever and bilateral tibial pain. Skeletal metastases cause bone pain which often worsens at night, being little relieved by sleep. It is the disturbance of the highly innervated periosteum that adds neurogenic attributes to the bone pain (12). Interestingly, the patient reported a sensation of “cold hands” while it was previously reported that osteoblastic lesions of unusual nature may present with increased numbness (13). Furthermore, other common signs of advanced bone metastases were not evident in this case, such as calcium irregularity, pathological fractures or spinal cord compression (3,12).
The differential diagnosis of diffuse sclerotic bone lesions consists of a variety of conditions such as breast and prostate cancer, osteoma and osteosarcoma, lymphoma, hyperparathyroidism, Paget’s Disease of the bone, vitamin D or fluoride intoxication, chronic osteomyelitis, congenital diseases such as osteopetrosis or pycnodysostosis, and vascular infarcts (i.e., sickle cell anemia) (5). However, the lack of additional features typical of each of these conditions, and a biopsy showing signet ring tumor cells in the stomach and the bone marrow ultimately confirmed the diagnosis.
The role of CA19-9 in the pathology and diagnosis of gastric carcinoma is not well defined. Although initially derived from colon cancer, measurement of CA19-9 in the blood is considered more specific for upper gastrointestinal malignancies, particularly pancreatic carcinoma (9). Furthermore, CA19-9 is known to occur in the tissues as a glycolipid, but in the serum as a mucin (9). Hence, we suggest that the observed increase in CA19-9 in this case is due to the overexpression of signet ring cells, which contain large amounts of mucin. Importantly, CA19-9 elevations were previously found not only in gastrointestinal tumors, but also in other conditions such as jaundice, cholangitis and non-gastrointestinal cancers, often mildly (14). These studies have undermined the efficacy of CA19-9 as a single diagnostic factor for gastrointestinal tumors and urged the need for identifying additional biomarkers for early identification of gastric cancer. It appears that until a deeper understanding is gained as to the conditions under which biomarkers like CA 19-9 are released, their role in the pathogenesis and diagnosis of cancers will be somewhat limited.
However, an interesting study recently conducted by Liu et al. (15) reported that a five microRNA signature was more accurate than CA19-9 for early diagnosis of gastric cancer by genome-wide serum micro RNA expression profiling. The idea of using microRNAs, which carry remarkable tissue specificity, to accurately identify cancer tissue origin, may greatly change our future approach for handling cancers of unknown primary origin (16). Moreover, applying novel techniques based on MicroRNAs profiling may help us understand better how tumors evolve and may shed light on the source of “traditional” biomarkers such as CA19-9. Lastly, it is suggested that applying microRNAs for more accurate tumor classification will offer improved management, treatment and prognosis for gastric carcinoma patients (16-20).
An important question raised by the findings in this case report is whether there is a common mechanism for all occurrences of osteoblastic metastasis, regardless of cancer origin. Recently, several factors have been identified as being important for bone formation in metastatic prostate cancer (the most common source of osteoblastic metastasis) including endothelin-1, TGF-β, TPA and fibroblast growth factors (FGFs) (1). Along with the understanding of the formation of bone metastasis in prostate cancer, novel treatments based on these mechanisms might be applicable also for patients with signet ring cell gastric carcinoma who present with osteoblastic metastasis. For example, it has been found that administration of Atrasentan, an Endothelin-1 receptor antagonist which blocks Endothelin-1 signaling pathway in osteoblasts, results in inhibition of bone formation and of tumor activity (21). In a number of other studies, osteoblasts were found to facilitate the progression of prostate tumor cells in the bone; and inhibition of these metastases by bio-chemicals such as strontium-89, samarium and radium-223, provided significant palliative treatment for patients and even prolonged their survival (22-25). Hence, we suggest that future research should examine whether novel treatments which were developed for prostate cancer with osteoblastic metastases might also facilitate the management and prognosis of patients with signet ring cell gastric carcinoma.
In conclusion, we have presented herein a rare case with diffuse skeletal osteoblastic metastases and a prominent increase of the tumor marker CA19-9 at presentation of gastric signet ring cell carcinoma. Clinicians should be conscious of the fact that this presentation might foreshadow the rare and grim possibility of gastric signet ring cell carcinoma.
Acknowledgement
The authors would like to express their deep participation in sorrow and gratitude to Mrs. Alona Faer for her willingness to share her beloved deceased husband’s medical information for the benefit of the medical community and future patients.
Footnote
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93. [PubMed]
- Guise TA, Mohammad KS, Clines G, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 2006;12:6213s-6216s. [PubMed]
- Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer 2005;5:21-8. [PubMed]
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell 2006;127:679-95. [PubMed]
- Carstens SA, Resnick D. Diffuse sclerotic skeletal metastases as an initial feature of gastric carcinoma. Arch Intern Med 1980;140:1666-8. [PubMed]
- Uchida T, Shikata T, Shimizu SI, et al. Gonadotropin and alkaline phosphatase producing occult gastric carcinoma with widespread metastasis of generalized bone. Cancer 1981;48:140-50. [PubMed]
- Chung YS, Choi TY, Ha CY, et al. An unusual case of osteoblastic metastasis from gastric carcinoma. Yonsei Med J 2002;43:377-80. [PubMed]
- Charhon SA, Chapuy MC, Delvin EE, et al. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma special reference to osteomalacia. Cancer 1983;51:918-24. [PubMed]
- Mann DV, Edwards R, Ho S, et al. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol 2000;26:474-9. [PubMed]
- Ni XG, Bai XF, Mao YL, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol 2005;31:164-9. [PubMed]
- Carpelan-Holmström M, Louhimo J, Stenman UH, et al. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res 2002;22:2311-6. [PubMed]
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-6249s. [PubMed]
- Courey RW. Osteoblastic lesions of unusual nature. JAMA 1972;219:377-8. [PubMed]
- Marrelli D, Caruso S, Pedrazzani C, et al. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg 2009;198:333-9. [PubMed]
- Liu R, Zhang C, Hu Z, et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer 2011;47:784-91. [PubMed]
- Rosenfeld N, Aharonov R, Meiri E, et al. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol 2008;26:462-9. [PubMed]
- Lakhani SR, Ashworth A. Microarray and histopathological analysis of tumours: the future and the past? Nat Rev Cancer 2001;1:151-7. [PubMed]
- Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834-8. [PubMed]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006;6:857-66. [PubMed]
- Ramaswamy S, Tamayo P, Rifkin R, et al. Multiclass cancer diagnosis using tumor gene expression signatures. Proc Natl Acad Sci U S A 2001;98:15149-54. [PubMed]
- Carducci MA, Padley RJ, Breul J, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol 2003;21:679-89. [PubMed]
- Quilty PM, Kirk D, Bolger JJ, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol 1994;31:33-40. [PubMed]
- Goyal J, Antonarakis ES. Bone-targeting radiopharmaceuticals for the treatment of prostate cancer with bone metastases. Cancer Lett 2012;323:135-46. [PubMed]
- Han SH, de Klerk JM, Tan S, et al. The PLACORHEN study: a double-blind, placebo-controlled, randomized radionuclide study with (186)Re-etidronate in hormone-resistant prostate cancer patients with painful bone metastases. Placebo Controlled Rhenium Study. J Nucl Med 2002;43:1150-6. [PubMed]
- Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 2001;357:336-41. [PubMed]