Hepatic resection, hepatic arterial infusion pump therapy, and genetic biomarkers in the management of hepatic metastases from colorectal cancer
Introduction
About 132,700 new cases of colorectal cancer (CRC) are diagnosed each year in the United States. The liver is the most common site of metastatic disease, with up to 60% of patients ultimately developing liver metastases (CRLM) (1). Fortunately, significant improvements have been made for patients with metastatic colorectal cancer (mCRC).
Initial reports of hepatic resection for CRLM demonstrated an unexpected, prolonged long-term survival (2). Long-term follow up documented the curative potential of hepatic resection for limited CRLM in 15 to 25% of patients (3). Up until the 1990’s, hepatic resections were fraught with significant blood loss, subsequent peri-operative complications, and a high mortality rate (4). Better understanding of hepatic anatomy, resection techniques, intraoperative anesthetic management, and postoperative care, have improved peri-operative outcomes. Currently, hepatic resection for CRLM is effective when performed at high volume specialty centers achieving a perioperative mortality rate of 1% (5,6). Parallel to this, evidence supports the use of hepatic artery infusion (HAI) of chemotherapy as an adjunct to managing CRLM. Likewise, our understanding of genetic aberration in CRLM emerges as important factor in treatment plans and prognosis.
In this review, we discuss surgical treatment and associated outcomes in the treatment of CRLM. In addition, the role and efficacy of HAI therapy are examined. Finally, we outline how genetic profiling and mutational analysis can impact management of this disease in this era of molecular-based targeted therapies.
Surgical management of CRLM
Resection for CRLM has been well established over the last three decades. Patient selection with preoperative multidisciplinary review and improved perioperative management make resection a safe and effective treatment modality for patients with operable CRLM.
Patients’ disease burden and future liver remnant are analyzed with cross-sectional imaging, volumetric studies, and evaluation of hepatic synthetic function. In general, patients with CRLM are considered resectable if their tumor burden can be removed with a negative margin while leaving a viable liver remnant that is able to drain bile and provide adequate synthetic function. Twenty percent of patients are estimated to have resectable disease at presentation (7).
Despite being technically resectable, outcomes are varied, and associated with a number of clinical and pathologic factors. Multivariate analysis of retrospective studies have shown that patient age, hepatic margin status, extrahepatic disease, number and size of tumors, CEA level, disease-free interval (DFI), and lymph node status of the primary tumor are associated with recurrence and survival after hepatic resection for CRLM (8,9). Many studies have combined these prognostic factors into clinical risk scores in attempts to improve prognostication. Stratifying patients into low and high-risk scores can predict survival following resection. In one example, a low-risk score was associated with a 60% 5-year survival while a high-risk score had an associated 14% 5-year survival. Despite effective stratification with clinical risk scores, patients with high-risk scores that undergo complete resection still have the potential for long-term survival and cure. These statistics underscore the need for better risk-stratification tools. The only factors that appear to make cure extremely unlikely, however, are a persistent positive hepatic margin and presence of extrahepatic disease (3,10). In summary, for patients with resectable liver-limited CRLM, the presence of adverse prognostic factors and high-risk scores do not preclude the potential for cure with complete resection and should not trump sound clinical judgment.
Hepatic parenchymal sparing techniques in lieu of extensive resections should now be routine in contemporary surgical management of CRLM and have been associated with significant improvements in perioperative outcomes (5,6). House et al. published a retrospective study of 1,600 consecutive patients who underwent resection for CRLM to determine the outcomes in two separate eras [1985-1998, 1999-2004]. The incidence of hemi-hepatectomy and wedge resections decreased in the latter era. Segmental resections are being performed more frequently with improved perioperative outcomes, and without jeopardizing oncologic principles (11). Historically, mortality following hepatic resection was high but now the 90-day mortality related to resection for CRLM is less than 1% in experienced high volume centers (5).
Despite 5-year survival rates of 20-50% following complete resection, recurrence rates approach 70-80% with long-term follow up (12). The high recurrence rates provide the rationale for treating microscopic disease with adjuvant chemotherapy, in an attempt to improve outcomes. Early randomized trials demonstrated that the addition of adjuvant 5-FU chemotherapy as compared to resection alone was not associated with improved progression-free (PFS), or overall survival (OS) (13).
The EORTC intergroup 40983 randomized trial evaluated perioperative FOLFOX for patients with limited and resectable CRLM (14,15). Patients were randomized to receive perioperative FOLFOX or surgery alone. The initial publication on this trial documented a significant 7.3% absolute increase in PFS. However, with longer term follow up, OS was not statistically different between the two groups. This trial demonstrated that perioperative FOLFOX chemotherapy may improve early PFS but was not associated with improved survival. While this trial was not powered to detect small differences, it ruled out a major impact on OS. However, this patient cohort was heterogenous. It is clear that select patients in each treatment group had durable survival while others did not. This again adds mounting evidence for the need of improved predictive factors and that CRLM is a heterogenous disease process.
In summary, multidisciplinary management that incorporates both patient and tumor-related factors should be performed in order to individualize treatment plans. Hepatic resection for CRLM is the standard of care for patients who are able to undergo operation and with resectable disease, due to associated long-term survival and potential for cure. Of those undergoing a potentially curative resection, survival is approximately 50% at 5-year, and the cure rate ranges from 20-25%, which is superior to chemotherapy alone (3). Unfortunately, the benefit of neoadjuvant and adjuvant systemic chemotherapy is not well understood in the context of curative surgery. The high recurrence rates after resection underscore the continued need for development of effective adjuvant therapies in patients undergoing resection of CRLM.
HAI pump therapy
Contemporary systemic therapies include 5-FU in combination with either oxaliplatin (FOLFOX), irinotecan (FOLFIRI) or both (FOLFOXIRI) (16-18). These provide response rates of 50% and median survivals of 16-24 months for untreated mCRC (17,19,20). Biologic agents targeting vascular endothelial growth factor (bevacizumab) or epidermal growth factor receptor (cetuximab) improve responses rates in select patients (21,22). Salvage with second and third line chemotherapeutic regimens once progression occurs provides diminutive benefit, with response rates no greater than 10% or 15% (23). These outcomes provide a benchmark with which to compare the efficacy of HAI chemotherapy.
HAI chemotherapy has been studied for decades (24,25). The therapy has not been universally embraced, perhaps because of the surgical training and expertise required for pump placement, the requirement for diligent and frequent follow-up, and the ability to recognize and manage complications. HAI chemotherapy requires establishment of a multi-disciplinary program consisting of a specialist surgeon, medical oncologist, interventional radiologist, gastroenterologist, nuclear medicine radiologist, technologists, and nursing staff.
The rationale for HAI therapy is based upon anatomic and pharmacologic principles. The hepatic arteries exclusively perfuse CRLM, while the portal vein and hepatic arteries jointly perfuse normal hepatocytes (26). The use of drugs that are extracted by the liver during first-pass metabolism results in high local concentrations of drug with minimal systemic exposure. Ensminger and colleagues showed that 94% to 99% of floxuridine (FUDR) is extracted by the liver during the first pass compared with 19% to 55% for 5-FU (27). In fact, mean tumor FUDR levels are increased 15-fold when the drug is injected via the hepatic artery (28). FUDR is therefore an ideal drug for HAI, providing a high hepatic concentration of drug with minimal systemic spill over and resultant toxicity. The development of an implantable infusion pump allowed for the safe administration of hepatic arterial chemotherapy in the outpatient setting (29).
Hepatic artery anatomy has a predilection for variation, with one third of patients possessing aberrant anatomy (30). Currently, computed tomography (CT) angiography provides accurate determination of patient anatomy. A surgeon experienced with dissection of the porta hepatis is required for HAI pump placement. The gastroduodenal artery (GDA) is the preferred conduit for the pump catheter, since other conduits are associated with increased rates of pump-related complications (30).
Hepatic arterial chemotherapy in first-line treatment of unresectable colorectal liver metastases
One of the first randomized trials of HAI therapy for unresectable CRLM was conducted at MSKCC (31). This prospective randomized trial compared HAI therapy with systemic chemotherapy using FUDR in both groups. Of the 99 enrolled patients, 2 complete responses and 23 partial responses (53%) were observed in the group undergoing HAI therapy, compared to 10 partial responses (21%) in the systemic chemotherapy group (P=0.001). The crossover rate from systemic chemotherapy to HAI therapy was 60%, of whom 25% subsequently underwent a partial response. The median survival for the HAI therapy and systemic chemotherapy groups was 17 and 12 months, respectively (P=0.424), despite the high cross over of the patients from the systemic chemotherapy group to the HAI therapy group.
The Cancer and Leukemia Group B (CALGB) completed trial 9481, which compared systemic chemotherapy with 5-FU/LV to HAI therapy using FUDR, LV, and dexamethasone (32). One hundred thirty-four patients were randomized without crossover. Most patients (70%) had greater than 30% liver involvement and 78% had synchronous metastases. Ninety-seven percent of patients had not received any chemotherapy. Response rates were significantly higher in the HAI therapy-only group (47% vs. 24%; P=0.012), but time to progression was not significantly different (5.3 vs. 6.8 months; P=0.8). Time to hepatic progression was significantly improved in the HAI therapy arm (9.8 vs. 7.3 months; P=0.017), median OS was significantly better in the HAI therapy arm (24.4 vs. 20 months; P=0.0034). At 3- and 6-month follow-up, physical functioning, as measured with quality of life instruments, was improved in the HAI therapy group.
A total of 10 randomized phase III trials comparing HAI to systemic therapy have been completed. Most of these demonstrate a higher response rate with HAI therapy as compared to systemic chemotherapy in patients with unresectable CRLM. Whether improved response rates translate into prolonged survival is unknown, and most trials were underpowered to detect survival differences. In addition, many of these studies allowed crossover to the HAI therapy. Many trials also used HAI with 5-FU, which is considered less effective than FUDR. Some trials included patients with extrahepatic disease, for which HAI alone is ineffective. Lastly, many trials utilized ports with high failure rates and inability to deliver therapy.
Two meta-analyses of the original seven trials were conducted and included more than 600 patients. The first confirmed the increased response rates seen with HAI therapy over systemic chemotherapy (41% vs. 14%) (33). A second meta-analysis published the same year found an absolute survival difference of 12.5% at 1 year (P=0.002) and 7.5% at 2 years (P=0.026) in favor of HAI therapy (34).
Combined hepatic arterial and systemic chemotherapy for treatment of unresectable colorectal liver metastases
Extrahepatic disease progression develops in 40% to 70% of patients who undergo HAI therapy for unresectable CRLM. Since HAI with FUDR results in minimal systemic exposure, combining HAI with FUDR and systemic chemotherapy was the next logical therapeutic strategy. Safi et al. studied whether intra-arterial FUDR alone or a combination of intra-arterial FUDR and IV FUDR given concurrently would improve survival (35). Response rates were 60% in both groups. However, the incidence of extrahepatic disease progression was significantly lower in patients who received combined systemic and hepatic therapy.
In a MSKCC phase I study, 36 patients with unresectable CRLM received HAI FUDR and systemic oxaliplatin plus irinotecan or oxaliplatin plus 5-FU/LV. Eighty-nine percent of patients were previously treated and 69% had previously received irinotecan. Both regimens were well tolerated, and response rates for the two groups were 90% and 88% (36). In a non-randomized study analyzing HAI therapy with FUDR and systemic irinotecan after cytoreduction of unresectable hepatic mCRC, 71 patients received therapy and were compared with a historic control group that received cytoreduction alone. Time to progression was 19 vs. 10 months, and median survival was 30.6 vs. 20 months for the HAI therapy vs. control groups, respectively (37). Similarly, a Japanese group examined HAI therapy with 5-FU and systemic irinotecan in previously treated patients and demonstrated response rates of 76.5%, with median OS of 20 months (38). Therefore, as compared systemic therapy alone, HAI therapy combined with modern systemic chemotherapy is associated with higher response rates.
Utilizing chemotherapy to convert unresectable patients to complete resection is an achievable goal of chemotherapy. Adam et al. presented their French experience of patients with unresectable CRLM. Of 1,104 patients considered unresectable at presentation, 12.5% were converted to surgical candidates with contemporary systemic cytotoxic chemotherapy (39). The patients who underwent resection realized a 3-year OS of 52%; a number far greater than the benchmark of 2 years for systemic therapy without resection. Importantly, most recurrences were extrahepatic providing the rationale for continued systemic chemotherapy. In a recent prospective phase II trial of HAI therapy and modern systemic chemotherapy combined with bevacizumab for patients with unresectable CRLM, 49 patients underwent evaluation of the conversion rate from unresectable liver metastases to complete resection as the primary outcome (40). Sixty-five percent of patients had received previous systemic chemotherapy. The median number of metastases was 14. The overall response rate was 76%. Importantly as depicted in a waterfall plot, most patients had a greater than 50% reduction in tumor volume (Figure 1). Such a dramatic improvement in tumor burden allows for resection to be considered. Twenty-three patients (47%) achieved conversion to resection at a median of 6 months from treatment initiation. Median OS and PFS were 38 and 18 months, respectively, with resection being the only factor associated with prolonged OS and PFS on multivariate analysis (3-year OS of 80% when resected compared with 26% in unresectable patients). Ten patients had no evidence of disease at the time of publication with a median follow up of 39 months. Importantly, a high biliary toxicity rate was found in the first 24 patients whose treatment included bevacizumab, but without any positive impact on outcome. As a result, bevacizumab is no longer used in HAI therapy combinations (41).
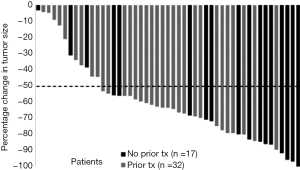
Moreover, Elias et al. presented their French experience of 87 patients with isolated CRLM between 1999 and 2003 who were treated with both HAI of oxaliplatin and systemic 5-FU. Importantly, 79% of patient had received prior contemporary systemic chemotherapy. Twenty-six percent of the cohort were converted to resectability and realized median OS of 42 months (42). Therefore, in two separate studies, HAI therapy converts unresectable patients to surgical candidates which confers long-term survival.
Adjuvant hepatic arterial chemotherapy following liver resection
Following resection of CRLM, at least 60% to 70% of patients recur at a median of 16 months (12). Patterns of recurrence are important to consider when devising adjuvant treatment strategies. At least half of all recurrences involve the liver, and, in one study, 64% of patients had their first site of recurrence in the liver (12). This provides rationale for targeting the liver as an adjunct to adjuvant systemic therapies.
There are four randomized trials evaluating adjuvant HAI chemotherapy following hepatic resection of CRLM. In an MSKCC study, 156 patients with resected hepatic metastases were randomized to either 6 months of systemic 5-FU/LV or systemic 5-FU/LV plus HAI therapy with FUDR (43). Randomization was performed intraoperatively after complete resection. Patients were stratified based on the number of metastases and prior treatment history. The primary endpoint was 2-year survival and was 86% in the combined-therapy group vs. 72% for those who received systemic chemotherapy alone (P=0.03), with median survival of 72.2 and 59.3 months, respectively. In an updated analysis, with a median follow-up of 10 years, OS was 41% in the HAI group versus 27% in the systemic chemotherapy only group (P=0.10) (8,44). Overall PFS was significantly greater in the combined-therapy group (31.3 vs. 17.2 months; P=0.02). The median hepatic PFS was not yet reached in the combined-therapy group, whereas it was 32.5 months in the monotherapy group (P<0.01).
In a German multi-institutional study, 226 patients were randomized to resection alone without systemic therapy or resection plus 6 months of HAI therapy with 5-FU/LV given as a 5-day continuous infusion every 28 days (20). The study was terminated early, due to an interim analysis suggesting a low chance of survival benefit with HIA therapy. The impact of HAI therapy in this study is difficult to assess because only 74% of patients assigned to HAI therapy received this treatment, and only 30% completed it. There was no difference in time to progression, time to hepatic progression, and median OS in an intention-to-treat analysis. When patients were analyzed “as treated”, time to hepatic progression (45 vs. 23 months) and time to progression or death (20 vs. 12.6 months) was improved in the HAI therapy arm. Despite this trial’s shortcomings, when analyzed appropriately it was still a positive trial showing HAI efficacy.
The intergroup study randomized 109 patients to resection alone without systemic therapy, or resection followed by 4 cycles of HAI therapy with FUDR and infusional systemic 5-FU, followed by systemic 5-FU (45). The endpoint was disease-free survival (DFS). The 4-year (DFS) (46% vs. 25%; P=0.04) and 4-year hepatic DFS (67% vs. 43%; P=0.03) favored HAI therapy but no difference was reported in median or 4-year OS between the groups when analyzed on an intention-to-treat basis.
Finally, a study conducted in Greece on 122 patients used mitomycin C, 5-FU, and interleukin (IL)-2 by both HAI therapy and the IV route vs. the IV route alone. The 2-year survival, 5-year survival, DFS, and hepatic DFS were all significantly longer for the HAI therapy plus systemic chemotherapy group (46).
The potential benefit of combination HAI FUDR therapy when combined with modern systemic chemotherapy in the adjuvant setting is unknown since no randomized trials addressing this have been performed. In a retrospective analysis, House and colleagues retrospectively compared 125 patients who underwent HAI therapy with FUDR with 125 consecutive similar patients who received modern systemic therapy alone, and noted an associated prolonged OS, hepatic recurrence-free survival (RFS), and disease-specific survival (DSS) with adjuvant combination HAI and systemic therapy; 75%, 48%, and 79%, vs. 55%, 25%, and 55%, respectively (P<0.01) (47). Therefore, despite contemporary cytotoxic chemotherapy, HAI FUDR continues to provide better outcomes for those with CRLM.
To further illustrate this point, a phase I trial combining adjuvant HAI FUDR with escalating doses of oxaliplatin and 5-FU was performed. Safety and feasibility were established and the 4-year OS and PFS were a very promising 88% and 50%. In a randomized phase II trial of patients treated with HAI FUDR and modern systemic chemotherapy (depending on prior treatment) randomized to receive bevacizumab or not, the 4-year OS was 85% (32,48).
In another study from France, 98 patients underwent curative resection of CRLM. Forty-four patients received combined HAI of oxaliplatin with systemic 5-FU. Fifty-four patients received contemporary systemic therapy alone. Three-year disease free survival was 33% compared to 5% (P=0.0001) for those treated with HAI oxaliplatin versus systemic alone. Additionally, OS showed a trend for improvement for those treated with HAI oxaliplatin (49).
A new review comparing patients treated with adjuvant HAI and systemic therapy after liver resection prior to 2003 or after 2003 show a 5-year survival of 56% and 80% for those treated before or after 2003, respectively (50).
In summation, these data show combination HAI and systemic chemotherapy therapy provide improved benefit compared to each alone. The findings provide the rationale for a randomized trial comparing adjuvant HAI therapy plus systemic chemotherapy versus modern systemic chemotherapy alone in the treatment of resected CRLM.
Genetic profiling and prognosis for colorectal liver metastases
Cancer is frequently associated with genetic aberrations. These aberrations lead to over or under production of proteins, which, in turn, lead to cellular transformation and autonomous growth potential. KRAS and BRAF mutations have emerged as important genetic aberrations affecting the management CRLM.
About 20% to 40% of CRC harbor mutations in KRAS (51-53). These mutations are conserved through all stages of a patient’s metastatic disease. This suggests that KRAS mutation may be a driving genetic alteration. KRAS mutations may also be prognostic (54). At MSKCC, a retrospective study was performed to determine the impact of KRAS mutation on DSS following hepatic resection for CRLM. KRAS mutation was independently associated with a worse DSS compared to wild-type tumors (2.6 vs. 4.8 years) (51). KRAS mutations were also associated with a short DFI and higher numbers of hepatic tumors. In a MD Anderson Cancer Center (MDACC) analysis, all patients undergoing hepatic resection for CRLM received preoperative contemporary cytotoxic chemotherapy and bevacizumab (53). Tumors harboring wild-type KRAS had fewer than 50% viable cells 58% of the time, compared to 38% of the time in mutated KRAS tumors. Hepatic and pulmonary recurrence rates were decreased for wild-type KRAS patients compared to mutated KRAS patients. These differences were associated with a prolonged OS for patients with wild-type KRAS tumors (81% compared to 52% at 3 years). In the Johns Hopkins experience, patients harboring mutated KRAS CRLM had a median RFS of 11 months compared 18 months for those with wild-type KRAS patients following curative resection of CRLM (52).
In another study, 169 patients with resected CRLM received adjuvant HAI therapy and systemic chemotherapy, of whom 118 were wild-type KRAS, and 51 had KRAS mutated tumors (55). The 3-year RFS for patients with wild-type KRAS tumors was 46%, compared with 30% for patients with mutated KRAS tumors (P=0.005). The 3-year OS was 95% vs. 81%, respectively. Interestingly, KRAS was an independent predictor of RFS (HR 1.9) on multivariate analysis. In summary, these data suggest that KRAS mutation is associated with an aggressive disease biology and worse outcome after resection of CRLM.
As stated, KRAS mutation is a poor prognostic factor for CRC. Additionally, KRAS mutation predicts a poorer outcome with systemic cytotoxic chemotherapy as illustrated in the MDACC and Johns Hopkins data. In the MSKCC experience, this holds true as well (Table 1). However, multimodality treatment for select patients utilizing resection, HAI, and systemic therapy appears to mitigate these poor outcomes. In an updated review of MSKCC experience, patients with CRLM and wild-type KRAS have a 3-year survival of 97% when treated with HAI FUDR and systemic therapy. Those with KRAS mutation realize a 3-year survival of 89% with HAI FUDR and systemic therapy. Both of these survivals are compelling evidence that HAI is providing benefit to those with CRLM above and beyond that provided by systemic therapies alone despite KRAS mutation status.

Full table
BRAF is a serine/threonine-protein kinase downstream in the signaling cascade from ras produced by the proto-oncogene BRAF. The gene is mutated in multiple tumors including CRC. In general, BRAF mutations portend worse outcome for patients with CRC. In a population-based analysis, OS for patients with mCRC harboring BRAF mutations was 8 months compared to 17 months for wild-type patients and was independently associated with worse outcome (HR 10.6, P <0.001) (56). In the context of metastasectomy for mCRC, the MSKCC experience was analyzed (57). Only 41% of patients with mutated BRAF had isolated liver disease as compared to 63% of those with wild-type BRAF. Metastases were more likely to be in the peritoneum (26%) or lung (12%) for BRAF mutants. Even in the context of curative metastasectomy, OS was 61% at 2 years for patients with BRAF mutations compared to 86% for wild-type. Despite resections with curative intent, BRAF mutation appears to be a poor prognostic factor.
Micro-array technology to assess mRNA expression in tumors has allowed investigators to study the prognostic impact of genetic expression signatures. Using high throughput RNA and genetic analysis methods, MSKCC has been able to improve accuracy of predicting 3-year outcomes following resection of CRLM by developing an expression molecular risk score (58). This molecular risk score was more prognostic of outcome compared to previously validated clinical risk scores. These results remain in their infancy and require external validation but provide the promise of improving our knowledge of CRLM management.
Conclusions
During the last three decades, there has been progressive improvement in the management of CRLM. Hepatic resection is performed with low risk at high-volume specialized centers, and has been established as the standard of care for resectable disease with associated prolonged survival and potential for cure. Likewise, systemic therapies have improved, with the advent of novel cytotoxic systemic chemotherapeutic agents. Furthermore, targeted therapies are now applied to contemporary drug regimens and have modestly improved outcomes in patients with mCRC. HAI chemotherapy has also evolved, and provides a unique and effective therapy both in the unresectable setting and as an adjuvant therapy following resection seemingly beyond that of systemic therapies alone. Multidisciplinary care for each patient with CRLM is crucial to orchestrate the multiple management strategies to extent survival. Combining clinical features with molecular profiling may provide superior prognostication for patients with CRLM. The promise of individualized therapy, tailored according to specific genetic mutations and disease patterns, is now being realized and continues to evolve.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Wanebo HJ, Semoglou C, Attiyeh F, et al. Surgical management of patients with primary operable colorectal cancer and synchronous liver metastases. Am J Surg 1978;135:81-5. [PubMed]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575-80. [PubMed]
- Ekberg H, Tranberg KG, Andersson R, et al. Major liver resection: perioperative course and management. Surgery 1986;100:1-8. [PubMed]
- Kingham TP, Correa-Gallego C, D'Angelica MI, et al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg 2015;220:471-9. [PubMed]
- House MG, Ito H, Gönen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg 2010;210:744-52, 752-5.
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [PubMed]
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254-62. [PubMed]
- Carpizo DR, Are C, Jarnagin W, et al. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol 2009;16:2138-46. [PubMed]
- Gold JS, Are C, Kornprat P, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 2008;247:109-17. [PubMed]
- de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 2009;250:440-8. [PubMed]
- Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 2008;26:4906-11. [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208-15. [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [PubMed]
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23-30. [PubMed]
- Price TJ, Segelov E, Burge M, et al. Current opinion on optimal systemic treatment for metastatic colorectal cancer: outcome of the ACTG/AGITG expert meeting ECCO 2013. Expert Rev Anticancer Ther 2014;14:1477-93. [PubMed]
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000;343:905-14. [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [PubMed]
- Lorenz M, Müller HH, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann Surg 1998;228:756-62. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [PubMed]
- Ramming KP, Sparks FC, Eilber FR, et al. Hepatic artery ligation and 5-fluorouracil infusion for metastatic colon carcinoma and primary hepatoma. Am J Surg 1976;132:236-42. [PubMed]
- Bern MM, McDermott W Jr, Cady B, et al. Intraaterial hepatic infusion and intravenous adriamycin for treatment of hepatocellular carcinoma: a clinical and pharmacology report. Cancer 1978;42:399-405. [PubMed]
- Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol 1954;30:969-77. [PubMed]
- Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol 1983;10:176-82. [PubMed]
- Sigurdson ER, Ridge JA, Kemeny N, et al. Tumor and liver drug uptake following hepatic artery and portal vein infusion. J Clin Oncol 1987;5:1836-40. [PubMed]
- Blackshear PJ, Dorman FD, Blackshear PL Jr, et al. The design and initial testing of an implantable infusion pump. Surg Gynecol Obstet 1972;134:51-6. [PubMed]
- Allen PJ, Stojadinovic A, Ben-Porat L, et al. The management of variant arterial anatomy during hepatic arterial infusion pump placement. Ann Surg Oncol 2002;9:875-80. [PubMed]
- Kemeny N, Daly J, Reichman B, et al. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med 1987;107:459-65. [PubMed]
- Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006;24:1395-403. [PubMed]
- Meta-Analysis Group in Cancer. Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst 1996;88:252-8. [PubMed]
- Harmantas A, Rotstein LE, Langer B. Regional versus systemic chemotherapy in the treatment of colorectal carcinoma metastatic to the liver. Is there a survival difference? Meta-analysis of the published literature. Cancer 1996;78:1639-45. [PubMed]
- Safi F, Bittner R, Roscher R, et al. Regional chemotherapy for hepatic metastases of colorectal carcinoma (continuous intraarterial versus continuous intraarterial/intravenous therapy). Results of a controlled clinical trial. Cancer 1989;64:379-87. [PubMed]
- Kemeny N, Jarnagin W, Paty P, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol 2005;23:4888-96. [PubMed]
- Bilchik AJ, Wood TF, Allegra D, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg 2000;135:657-62; discussion 662-4. [PubMed]
- Shitara K, Munakata M, Kudo T, et al. Combination chemotherapy with hepatic arterial infusion of 5-fluorouracil (5-FU) and systemic irinotecan (CPT-11) in patients with unresectable liver metastases from colorectal cancer. Gan To Kagaku Ryoho 2006;33:2033-7. [PubMed]
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644-57; discussion 657-8. [PubMed]
- DʼAngelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg 2015;261:353-60. [PubMed]
- Cercek A, D'Angelica M, Power D, et al. Floxuridine hepatic arterial infusion associated biliary toxicity is increased by concurrent administration of systemic bevacizumab. Ann Surg Oncol 2014;21:479-86. [PubMed]
- Goéré D, Deshaies I, de Baere T, et al. Prolonged survival of initially unresectable hepatic colorectal cancer patients treated with hepatic arterial infusion of oxaliplatin followed by radical surgery of metastases. Ann Surg 2010;251:686-91. [PubMed]
- Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999;341:2039-48. [PubMed]
- Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med 2005;352:734-5. [PubMed]
- Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol 2002;20:1499-505. [PubMed]
- Lygidakis NJ, Sgourakis G, Vlachos L, et al. Metastatic liver disease of colorectal origin: the value of locoregional immunochemotherapy combined with systemic chemotherapy following liver resection. Results of a prospective randomized study. Hepatogastroenterology 2001;48:1685-91. [PubMed]
- House MG, Kemeny NE, Gönen M, et al. Comparison of adjuvant systemic chemotherapy with or without hepatic arterial infusional chemotherapy after hepatic resection for metastatic colorectal cancer. Ann Surg 2011;254:851-6. [PubMed]
- Kemeny N, Capanu M, D'Angelica M, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol 2009;20:1236-41. [PubMed]
- Goéré D, Benhaim L, Bonnet S, et al. Adjuvant chemotherapy after resection of colorectal liver metastases in patients at high risk of hepatic recurrence: a comparative study between hepatic arterial infusion of oxaliplatin and modern systemic chemotherapy. Ann Surg 2013;257:114-20. [PubMed]
- Kemeny N, Chou J, Boucher T, et al. Improvement in long-term survival in patients with metastatic colorectal cancer (CRC) after liver resection with modern chemotherapy and hepatic arterial infusion (HAI). J Clin Oncol 2015;33:abstr 3563.
- Nash GM, Gimbel M, Shia J, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol 2010;17:572-8. [PubMed]
- Karagkounis G, Torbenson MS, Daniel HD, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer 2013;119:4137-44. [PubMed]
- Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg 2013;258:619-26; discussion 626-7. [PubMed]
- Ahnen DJ, Feigl P, Quan G, et al. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res 1998;58:1149-58. [PubMed]
- Kemeny NE, Chou JF, Capanu M, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer 2014;120:3965-71. [PubMed]
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623-32. [PubMed]
- Yaeger R, Cercek A, Chou JF, et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer 2014;120:2316-24. [PubMed]
- Ito H, Mo Q, Qin LX, et al. Gene expression profiles accurately predict outcome following liver resection in patients with metastatic colorectal cancer. PLoS One 2013;8:e81680. [PubMed]


