Changes in neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios during chemoradiation predict for survival and pathologic complete response in trimodality esophageal cancer patients
Introduction
Esophageal cancer is the sixth leading cause of cancer death worldwide, and the incidence is rapidly increasing. In the United States, the estimated incidence of new esophageal cancer diagnoses in 2015 is 16,980, leading to an estimated 15,590 deaths (1). Esophageal cancer has a particularly poor prognosis, with estimated 5-year overall survival (OS) ranging from 15% to 25%. Surgery is common in patients with locally advanced disease, and prospective randomized data indicate that neoadjuvant chemoradiation therapy (CRT) improves OS (2).
Inflammation is now recognized as playing a major role in carcinogenesis (3). In esophageal cancer, chronic inflammation is believed to be a major risk factor for development of squamous cell carcinoma (SCC) and adenocarcinoma (AC) (4). A number of studies focusing on different cancer types have identified specific prognostic inflammatory markers, including the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR). Elevated NLR has been associated with poorer prognoses in colorectal carcinoma (5), esophageal cancer (6), and head and neck cancer (7). Elevated PLR has been associated with poorer prognoses in pancreatic cancer (8), colorectal cancer (9), gastric cancer (10), and others (11).
Most published studies have analyzed the prognostic value of these markers as assessed in the pretreatment setting rather than as assessed during or after treatment. The prognostic value of NLR and PLR during trimodality therapy for esophageal cancer has not been reported and is the focus of this retrospective study.
Methods
Patients
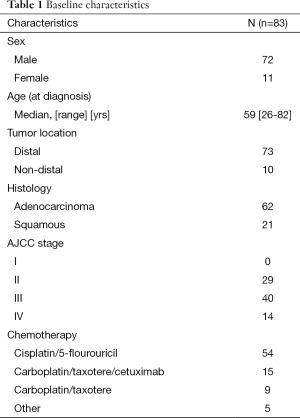
We conducted a search of an IRB-approved database from the Department of Radiation Oncology at our institution to identify patients who underwent trimodality therapy (neoadjuvant CRT followed by surgery) for locally advanced esophageal cancer from March 2000 to April 2012. Inclusion criteria for the study included biopsy confirmation of AC or SCC of the esophagus or gastroesophageal junction (GEJ), no evidence of distant metastasis, completion of neoadjuvant CRT followed by surgical resection with curative intent, and availability of complete blood cell counts (CBC) with differential at all three time points (TPs) (as defined later in this section). We identified 187 trimodality esophageal patients in our database, of whom 83 (72 men, 11 women) had the required CBC data at each TP required for inclusion in this study. The median age at diagnosis was 59 years (range, 26-82 years). Most patients (88%) had distal esophageal involvement, AC histology (75.7%), stage III or IV tumors (91.6%), and clinical nodal involvement (60.2%) (Table 1).
Full table
Treatment characteristics
Most patients received cisplatin 75 mg/m2 on days 1 and 29, with 5-fluorouracil 1,000 mg/m2 given on days 1-4 and 29-32 as a continuous infusion. Two- or three-dimensional radiotherapy was used before 2008, and intensity-modulated radiation therapy (IMRT) was primarily utilized starting in 2008. Gross tumor volume (GTV) was defined using information obtained from endoscopic ultrasound, CT, and/or positron emission tomography. Prior to 2008, the target volume consisted of an expansion of 5 cm proximally and 2 cm distally around the GTV. Beginning in 2008, the clinical target volume (CTV) was generated using a 4-cm superior and inferior and 1-cm axial expansion around the GTV and 1-cm isotropic expansion around grossly involved lymph nodes. Elective regional lymph node regions included within the CTV depended on the location of the primary tumor: celiac nodes for distal thoracic esophageal and GEJ tumors, paraesophageal nodes for midthoracic esophageal tumors, and bilateral supraclavicular nodes for upper thoracic and cervical esophageal tumors. Starting in 2008 the PTV was created using a 0.5-cm expansion on the CTV using daily image guidance. IMRT was used exclusively in more recent years, typically with 5 coplanar beams (12). Three-dimensional conformal radiation therapy was typically delivered using antero-posterior/posterior-anterior (AP/PA) beam arrangements up to 36 Gy, followed by an off-cord approach using AP and posterior oblique beams. Patients were prescribed a total dose of either 50.4 Gy (n=31) or 56.4 Gy (n=52). Patients who were prescribed 56.4 Gy were treated on a clinical trial evaluating dose escalation using IMRT. The most common concurrent chemotherapy regimen was cisplatin with 5-fluorouracil (66%), followed by paclitaxel with carboplatin and cetuximab (18%).
NLR and PLR measurements
CBCs with differentials were recorded at specified TPs. The first (TP1) was after diagnosis and prior to start of neoadjuvant CRT. The second (TP2) was after completion of neoadjuvant CRT and prior to surgery. The last (TP3) was after surgery. Absolute platelet, neutrophil, and lymphocyte counts were recorded at each TP. The NLR at each TP was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The PLR at each TP was calculated by dividing the absolute platelet count by the absolute lymphocyte count. Differences in NLR and PLR were calculated as follows: TP2 – TP1, TP3 – TP1, and TP3 – TP2. In addition to calculating differences in NLR and PLR values, relative changes in NLR and PLR were calculated as follows: TP2 ÷ TP1 (TP2/TP1), TP3 ÷ TP1 (TP3/TP1), and TP3 ÷ TP2 (TP3/TP2).
Statistical analysis
OS was defined as the interval from the date of diagnosis to date of death or last follow-up. Progression-free survival (PFS) was defined as the time interval from the date of diagnosis to date of distant or local recurrence. If no progression was noted, PFS was determined from the date of last follow-up or death. Pathologic complete response (PCR) was defined as histopathologic absence of tumor in the resected specimen. All factors significant on univariate analyses with a P<0.15 were evaluated in multivariate analyses. All statistical analyses were conducted using R software (13).
Results
Patient and treatment characteristics
All patients underwent esophagectomy following completion of neoadjuvant CRT. Persistent nodal disease was noted in 26 patients (31%), and only two patients (2.4%) had positive margins. The median time from completion of neoadjuvant CRT to surgery was 54 days (range, 38-221 days). PCR was noted in 30 patients (36%), gross residual disease was noted in 44 patients (53%), and 9 patients (11%) had microscopic residual disease (Table 1).
NLR and PLR characteristics
The median NLR and PLR at TP1 were 3.3 (range, 0.25-34.25) and 157.2 (range, 56.25-569), respectively. The median number of days between the TP1 lab values and start of neoadjuvant CRT was 10 days (range, 1-53 days). The median NLR and PLR at TP2 were 12 (range, 0.75-92.0) and 645 (range, 16.25-5,480), respectively. The median NLR and PLR at TP3 were 11.5 (range, 1.0-58.5) and 391.7 (range, 125.7-1,760), respectively. TP3 lab values were obtained at a median of 21 days (range, 3-100 days) after surgery.
Overall survival (OS)
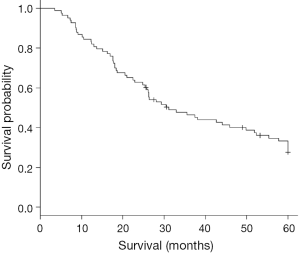
The median OS was 28.9 months for the entire cohort (Figure 1). On univariate analysis, worse OS was associated with increasing age as a continuous variable. On multivariate analysis, worse OS was associated with increasing age as a continuous variable, age >54 years, pathologic lymph node involvement, and a NLR increase TP3/TP2 >1.7 and as a continuous variable. Improved OS was associated with PLR >250 after resection and TP3 – TP2 PLR >609.2 (Table 2).

Full table
Progression-free survival (PFS)
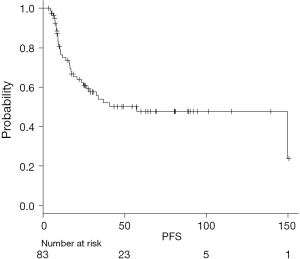
On univariate analysis, improved PFS was associated with distal tumor location, NLR >6.3 at TP3 and PLR >245.3 at TP3 (Figure 2). Worse PFS was associated with NLR >36 and PLR >429.7 at TP2 (Table 3).

Full table
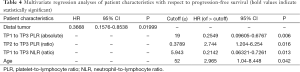
On multivariate analysis, improved PFS was associated with distal tumor location, PLR increase >19 between TP2 and TP1, and NLR increase TP3/TP1 >5.94. On multivariate analysis, worse PFS was associated with NLR decrease >28.25 between TP2-TP1, a decrease in PLR (TP3/TP2) >0.38, and age >52 years at time of diagnosis (Table 4).
Full table
Pathologic complete response (PCR)
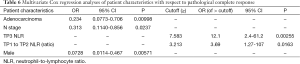
Higher rates of PCR were associated with a TP1 PLR >201.5 and TP2 NLR >10.55. Higher odds of PCR were associated with use of cisplatin/5-fluorouracil when compared with other chemotherapy regimens. Lower odds of PCR were associated with AC histology and pathologic nodal involvement. A higher probability of PCR was associated with TP2/TP1 NLR >3.21 (Tables 5,6).

Full table
Full table
Discussion
NLR and PLR are easily accessible and readily available to measure systemic inflammation. NLR reflects both neutrophilia, resulting from the inflammatory response caused by cancer, and lymphopenia, which may result from cortisol-induced stress response or malnutrition (14). A low lymphocyte count may reflect the inability of the body to mediate effective cell-mediated immunity to initiate cancer cell death (15). Furthermore, chemoradiation is thought to suppress immune response by decreasing certain immune cell count (16). Higher NLR, therefore, is associated with worse prognosis. Thrombocytosis is also believed to result from the inflammatory properties of tumor. Pro-inflammatory mediators [such as interleukin (IL)-1, IL-2, and IL-6] released by inflammation are believed to stimulate megakaryocytes, resulting in an elevated platelet count (17). Although the comparative significance of NLR and PLR is unclear, Feng et al. suggested that PLR is superior to NLR in predicting OS in patients with esophageal SCC (17). Ji et al. suggested that NLR might have more prognostic value in patients undergoing neoadjuvant CRT for locally advanced esophageal SCC (18).
To our knowledge, this is the first study to analyze the significance of NLR and PLR values at several TPs throughout treatment in esophageal cancer patients undergoing neoadjuvant CRT followed by esophagectomy. We demonstrate that not only did pretreatment NLR and PLR values predict for clinical outcomes, as has been shown by others, but that NLR and PLR changes after CRT and surgery were also significant predictors. For example, the most significant predictors for OS on multivariate analysis were changes in NLR and PLR between TP3 and TP2.
With few exceptions, the published data suggest that elevated preoperative NLR is associated with worse outcomes. Sato et al. evaluated NLR in advanced esophageal cancer patients undergoing neoadjuvant chemotherapy and showed that decreased PCR corresponded with elevated pretreatment NLR (19). Our study found no such association. However, for unclear reason, a higher probability of PCR was associated with an NLR increase between TP2 and TP1. Sharaiha et al. evaluated preoperative NLR in esophageal patients who underwent esophagectomy and showed that elevated NLR is associated with worse disease-free survival and OS (6). However, Dutta et al. evaluated preoperative NLR and PLR in 112 patients undergoing resection for esophageal cancer and did not find NLR to be a significant predictor of outcomes (14).
A smaller number of studies have also looked at the prognostic value of PLR in patients with esophageal cancer. Feng et al. evaluated preoperative NLR and PLR in 483 patients undergoing esophagectomy for esophageal SCC (17). That study demonstrated that an elevated preoperative PLR was a superior predictor of OS. Dutta et al., however, found that PLR was not associated with prognosis (14). Our study found both PLR and NLR to be predictors of OS, with similar prognostic values.
Radiation therapy and chemotherapy have well-known hematologic effects and, therefore, are expected to have a direct effect on NLR and PLR. However, little data are available on NLR and/or PLR after neoadjuvant therapy in esophageal cancer patients who also undergo surgery. In esophageal SCC patients who received neoadjuvant chemotherapy followed by surgery, Ji et al. evaluated the significance of NLR and PLR before and after chemotherapy, but radiation therapy was not used. The authors reported that not only was prechemotherapy NLR an independent prognostic factor for OS but also that patients who maintained a low NLR and PLR throughout chemotherapy experienced better OS (18). Our data also suggest that higher NLR values throughout treatment are associated with worse OS. A strong association between higher PLR values and improved PFS was noted in our study, although the reason is unclear.
This study has several limitations. The potential for selection bias exists, as in all retrospective studies. Also, evaluation of immune response within the tumor would be ideal, rather than analysis of cells in peripheral blood as in this study. However, tumor samples had not been obtained among these patients during neoadjuvant therapy.
Conclusions
Our data are the first to demonstrate that changes in NLR and PLR after CRT and after surgery may be better predictors than pretreatment NLR and PLR values. Such changes are likely induced by chemotherapy and radiation therapy. Prospective evaluation of NLR and PLR is warranted to further elucidate the utility of such prognostic markers in esophageal cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Fridman WH, Galon J, Pagès F, et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 2011;71:5601-5. [PubMed]
- Bird-Lieberman EL, Fitzgerald RC. Early diagnosis of oesophageal cancer. Br J Cancer 2009;101:1-6. [PubMed]
- Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181-4. [PubMed]
- Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011;18:3362-9. [PubMed]
- Rassouli A, Saliba J, Castano R, et al. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck 2015;37:103-10. [PubMed]
- Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 2009;197:466-72. [PubMed]
- Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012;17:216-22. [PubMed]
- Aliustaoglu M, Bilici A, Ustaalioglu BB, et al. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol 2010;27:1060-5. [PubMed]
- Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One 2014;9:e101119. [PubMed]
- Boggs DH, Tarabolous C, Morris CG, et al. Analysis of pathological complete response rates with paclitaxel-based regimens in trimodality therapy for esophageal cancer. Dis Esophagus 2015;28:619-25. [PubMed]
- R Core Team. A languange and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/
- Dutta S, Crumley AB, Fullarton GM, et al. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg 2011;35:1861-6. [PubMed]
- Lissoni P, Brivio F, Fumagalli L, et al. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers 2004;19:135-40. [PubMed]
- Parikh F, Duluc D, Imai N, et al. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer Res 2014;74:7205-16. [PubMed]
- Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 2014;12:58. [PubMed]
- Ji WH, Jiang YH, Ji YL, et al. Prechemotherapy neutrophil : lymphocyte ratio is superior to the platelet : lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapy. Dis Esophagus 2015. [Epub ahead of print]. [PubMed]
- Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 2012;36:617-22. [PubMed]