Histopathologic tumor response after induction chemotherapy and stereotactic body radiation therapy for borderline resectable pancreatic cancer
Introduction
Despite advances in treatment over the past several decades, pancreatic ductal adenocarcinoma (PDAC) remains a devastating disease with limited survival for the majority of patients. Only those who undergo margin-negative (R0) surgical resection have a reasonable likelihood of long-term disease control and potential cure. Unfortunately, only 15-20% of patients have resectable disease at diagnosis with the remainder presenting with distant metastatic or locally advanced tumors. Even after successful resection the expected 5-year survival is less than 20% (1).
Borderline resectable pancreatic cancer (BRPC) patients are unlikely to undergo R0 resection at initial diagnosis although they are more likely to do so after preoperative chemotherapy and radiation therapy (RT) (2,3). Preoperative RT for BRPC is typically delivered using conventional fractionation. In recent years stereotactic body radiation therapy (SBRT) has become increasingly used for BRPC based on emerging data suggesting significant local effect with minimal toxicity (4-7). Furthermore, SBRT is completed in no more than 5 fractions, thus providing a much shorter overall treatment time and increased patient convenience compared to conventionally fractionated RT that is delivered over 5.5 weeks. Prospective evaluation of SBRT for BRPC is currently ongoing (NCT01992705, NCT01360593) in an attempt to verify its safety and efficacy in ultimately achieving an R0 resection.
Several important prognostic factors have been identified in resected PDAC cancer patients including margin status (8,9), lymph node involvement (10-12), and CA 19-9 level (13-15). The degree of histopathologic tumor response, or tumor regression grade (TRG), after preoperative therapy is another emerging prognostic factor and has been evaluated in patients with various cancers including those of the head and neck, rectum, and esophagus (16-20). Emerging data suggest that TRG is also a significant independent prognostic factor for PDAC after chemotherapy alone or chemoradiation (21-23). A retrospective study from MD Anderson Cancer Center (MDACC) described a significant correlation between TRG score and survival in 223 pancreatic cancer patients who received neoadjuvant chemoradiation (21). In that study, patients with less residual tumor had more favorable pathologic findings (less frequent positive margins and lymph node involvement) and improved survival on multivariate analysis. One strategy to improve histopathologic tumor regression is through RT dose intensification, which can be achieved using SBRT (22). We evaluated the effect of TRG on clinical outcomes in BRPC patients who received preoperative therapy including SBRT.
Methods
After obtaining Institutional Review Board approval, a pancreas cancer database maintained in the Department of Radiation Oncology was queried to identify BRPC patients treated with induction chemotherapy followed by SBRT at our institution between 2009 and 2012. As was previously reported, SBRT was delivered to the primary tumor using a 5-fraction approach using 5-6 Gy per fraction including dose painting the region of vessel abutment by tumor up to 8 Gy per fraction provided all normal tissue constraints could be respected, particularly for the stomach, duodenum, and small bowel (4). Fiducial markers were routinely used for target delineation and daily image guidance. Patients were treated either with respiratory gating or abdominal compression. Four weeks after treatment all patients underwent restaging PET/CT and pancreas protocol CT scans. Patients determined to be fit for surgery, without evidence of metastatic disease, were recommended to have surgical exploration and surgical resection, if appropriate. Surgery was performed typically between 6-8 weeks after SBRT completion. Only patients who underwent definitive surgery with curative intent were included in this analysis.
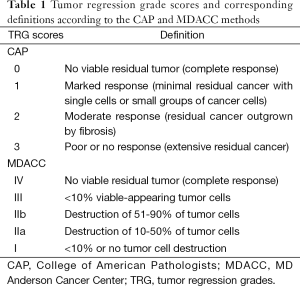
Resected specimens were examined at the time of grossing to identify the tumor site. If no residual grossly visible tumor was identified, the entire residual area with fibrosis was submitted for evaluation. All of the slides were reviewed and one expert pancreatic pathologist (B.A.C.) reviewed all pancreatic resection specimens. The amount of residual tumor was assessed by evaluating all of the sections derived from the tumor bed. Each patient’s tumor specimen was assigned two TRG scores (Table 1), one from the College of American Pathologists (CAP) Cancer Protocols and the other from the MDACC. The CAP method is a 4-tiered system in which a grade of 0 indicates a pathologic complete response (pCR) and a grade of 3 indicates either poor or no response (24). The published MDACC method used in this study is a 5-tiered system in which a pCR is indicated by a grade of IV, and no to minimal (<10%) tumor cell destruction is indicated by a grade of 1 (25).
Full table
Each patient’s TRG scores, using both scoring methods, were evaluated with respect to progression free survival (PFS) and overall survival (OS) using the Kaplan Meier method. OS was determined from the start of induction chemotherapy to date of death or last follow up if the patient was still alive. PFS was determined from the start of induction chemotherapy to the date of first recurrence, death, or last follow up.
Results
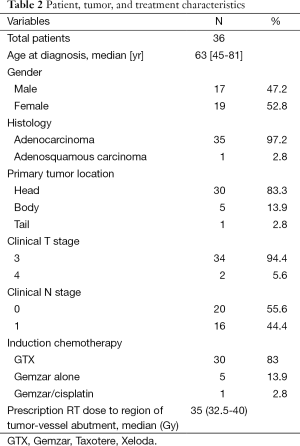
Of a total 57 BRPC patients who completed induction chemotherapy and SBRT and underwent surgical resection, the initial 36 consecutive patients were included in this study. Patient and tumor characteristics are described in Table 2. The median age was 63 years (range, 45-81 years) and 18 patients were female (51.4%). The pancreatic head was involved in 30 patients (85.7%) with the remainder involving the body/tail (14.3%). Most were clinically staged as T3 (91.4%) and N0 (54.3%).
Full table
The most commonly delivered induction chemotherapy (85.7%) was a combination of gemcitabine, docetaxel, capecitabine (GTX) and it was generally well tolerated as the majority of patients received the prescribed chemotherapy regimen (82.9%) consisting of Gemzar, Taxotere and Xeloda (GTX). All patients completed the prescribed SBRT course, with none experiencing significant acute toxicity. A median dose of 35 Gy (range, 32.5-40 Gy) was prescribed to the region of vessel abutment while the remainder of the primary tumor was treated to a median dose of 30 Gy (range, 25-30 Gy). Most patients underwent a pancreaticoduodenectomy (88.6%) after a median 5.6 weeks (range, 4.6-9.1 weeks) from SBRT completion. Of the 36 total patients, 35 were resected with negative surgical margins (97.2%).
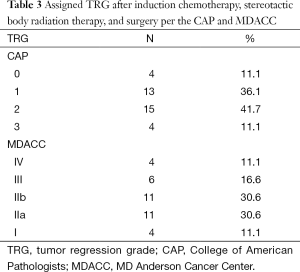
Most patients had moderate to significant tumor regression. Complete response was present in four patients and was reflected by CAP grade 0 and MDACC grade IV. CAP grades of 0, 1, 2, and 3 were assigned to 4 (11.1%), 13 (36.1%), 15 (41.7%), and 4 (11.1%) patients, respectively. MDACC grades of IV, III (M), IIB, IIA, and I were assigned to 4 (11.1%), 6 (16.6%), 11 (30.6%), 11 (30.6%), and 4 (11.1%) patients, respectively (Table 3). Therefore only a minority of patients (11.1%) per either grading system had a poor response (CAP grade 3 or MDACC grade I).
Full table
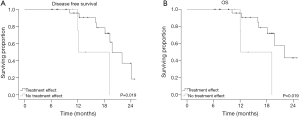
Median follow-up was 13.8 months (range, 6.1-24.8 months). Median OS was 22.5 months and median PFS was 14.9 months. Improved treatment response (higher score) according to the MDACC method trended towards superior PFS (P=0.061), but not OS (P=0.134). Any histopathologic treatment effect (IIA-IV vs. I) according to MDACC method predicted for improved OS (22.9 vs. 14.5 months) and PFS (15 vs. 7.4 months; both P=0.019) (Figure 1). We found no significant relationship between CAP grade and either OS or PFS. All four patients with a pCR were alive and without evidence of disease at the time of last follow up. Lastly, no local recurrences have been reported in any patient.
Discussion
While most PDAC patients have unresectable disease at initial presentation, those classified as having BRPC who undergo R0 resection after preoperative chemoradiation potentially can have long-term disease control (2,3). While prospective data support SBRT for PDAC patients with locally advanced unresectable disease (26-29), emerging retrospective evidence for BPRC are encouraging that preoperative regimens including SBRT may effectively increase the likelihood of complete resection with minimal toxicity and in a much more patient-friendly timeframe as compared to conventionally fractionated RT (4,5). Although no prospective SBRT data for BRPC have been published, several clinical trials are ongoing. These trials include one at our institution (NCT01992705), anticipated to support the continued use of pancreatic SBRT.
Several independent prognostic factors have been identified for patients with PDAC including margin status (8,9), lymph node involvement (10-12), and CA 19-9 level (13-15). The degree of histopathologic response after preoperative therapy, or TRG, is another prognostic factor that has been shown to be significantly related to clinical outcomes for various cancers, especially of the gastrointestinal tract (17,20,21,24,25,30-32). In fact, some studies have suggested that the prognostic significance of histopathologic tumor response warrants incorporation of TRG into staging systems for patients who have received preoperative therapy (33-35). Despite this, TRG scores are infrequently used for clinical decision-making, in large part due to the many TRG scoring methods that exist, consequentially leading to a lack of standardization. These different methods essentially utilize varying thresholds for the ratio between residual tumor versus tumor that has been replaced by fibrous or fibromatous granulation tissue (21,22,31,32,36-38). It is clear that validation studies are needed to clarify which method should become the standard.
We evaluated TRG in this study using the methods published by the CAP and MDACC. There were several reasons for this. First, as has been previously mentioned, data is lacking to conclude which method is the “best” among the multiple that have been developed. Thus, we decided to report scores from two separate scoring methods to have a higher likelihood of showing a significant correlation between at least one method with our clinical outcomes. Second, we selected the CAP and MDACC approaches because they are two of the most commonly reported in the literature, which would make our results more generalizable.
Induction chemotherapy and SBRT resulted in most patients having greater than minimal histopathologic effect (~90%) according to CAP (grade 0-2) and MDACC (grade IV-IIA) criteria. Complete response was achieved in 4 patients (11%). We could not determine the relative contribution of chemotherapy vs. SBRT on tumor regression. However, compared to a study from MDACC in which SBRT was not given it is interesting that a higher percentage of significant response defined as CAP grade 0-1 (47% vs. 19%) or MDACC grade IV-III (28% vs. 19%) was seen in our patients who received SBRT (21). It is important to note that a significant number of patients in the MDACC study received chemoradiation with 30 Gy in 10 fractions, which has a lower biologically effective dose (BED10=39 Gy) compared to 50.4 Gy in 28 fractions (BED10=59.5 Gy) and 35 Gy in 5 fractions (BED10=59.5 Gy), which was the median dose delivered in our study to the region of vascular involvement (39,40). We note that 11 patients in our study safely received up to 40 Gy in 5 fractions (BED10=72 Gy) to the region of vascular involvement. Finally, we recognize that we cannot draw any conclusions from this comparison given our fairly small patient number and the heterogeneity in chemotherapy between studies. However it is plausible that increased tumor regression may be achieved through dose escalation using SBRT.
We found no correlation between OS or PFS and CAP grade. On the other hand, we observed a trend towards superior PFS (P=0.06) with increasing histopathologic response according to the MDACC method. The minority of patients per MDACC criteria (n=4) had a poor response to preoperative therapy, which was associated with significantly worse OS and PFS (both P=0.02). Reasons for limited response after intense multi-agent chemotherapy and SBRT are not known, but could be in part related to the poor inherent radiosensitivity of those tumors (39). Why we found a correlation between the MDACC but not the CAP grading method is also not obvious, but could be because the MDACC grading is 5-tiered (vs. the 4-tiered CAP method) and therefore a finer level of distinction could be made between patients with a partial response. As was previously noted, there was good agreement between patients who had minimal or poor response (CAP grade 3, MDACC grade I). The MDACC method is also much more objective, requiring TRG scores to be assigned based on the destruction of a certain percentage of tumor cells. On the other hand, the CAP method is largely subjective, requiring the pathologist to determine TRG based on a “marked”, “moderate”, or “poor” treatment response.
We recognize that there are several limitations of this study including its retrospective design, small patient number, and relatively limited follow up. We attempted to minimize selection bias by evaluating an initial group of consecutive BRPC patients treated at our institution using SBRT. We also accounted for interobserver bias in TRG assessment by having only one pathologist with expertise in PDAC (B.A.C.) evaluates all tumor specimens.
This is the first study to characterize TRG in BRPC patients after undergoing preoperative therapy with induction chemotherapy followed by SBRT. While we could not isolate TRG as a result of SBRT alone, we believe that SBRT likely contributed significantly to the excellent overall tumor responses that we observed. It remains unclear if the effect of SBRT vs. standard fractionation RT differs for BRPC. Even if tumor regression is similar between these two dose fractionation strategies, there are increasingly apparent clinical advantages of SBRT that warrant its continued evaluation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004;363:1049-57. [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 846-8. [PubMed]
- Kharofa J, Tsai S, Kelly T, et al. Neoadjuvant chemoradiation with IMRT in resectable and borderline resectable pancreatic cancer. Radiother Oncol 2014;113:41-6. [PubMed]
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516-22. [PubMed]
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. [PubMed]
- Moningi S, Dholakia AS, Raman SP, et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann Surg Oncol 2015;22:2352-8. [PubMed]
- Rajagopalan MS, Heron DE, Wegner RE, et al. Pathologic response with neoadjuvant chemotherapy and stereotactic body radiotherapy for borderline resectable and locally-advanced pancreatic cancer. Radiat Oncol 2013;8:254. [PubMed]
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567-79. [PubMed]
- Tummala P, Howard T, Agarwal B. Dramatic Survival Benefit Related to R0 Resection of Pancreatic Adenocarcinoma in Patients With Tumor ≤25 mm in Size and ≤1 Involved Lymph Nodes. Clin Transl Gastroenterol 2013;4:e33. [PubMed]
- Moghanaki D, Mick R, Furth EE, et al. Resection status, age and nodal involvement determine survival among patients receiving adjuvant chemoradiotherapy in pancreatic adenocarcinoma. JOP 2011;12:438-44. [PubMed]
- House MG, Gönen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg 2007;11:1549-55. [PubMed]
- You DD, Lee HG, Heo JS, et al. Prognostic factors and adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. J Gastrointest Surg 2009;13:1699-706. [PubMed]
- Kinsella TJ, Seo Y, Willis J, et al. The impact of resection margin status and postoperative CA19-9 levels on survival and patterns of recurrence after postoperative high-dose radiotherapy with 5-FU-based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol 2008;31:446-53. [PubMed]
- Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 2006;24:2897-902. [PubMed]
- Smith RA, Bosonnet L, Ghaneh P, et al. Preoperative CA19-9 levels and lymph node ratio are independent predictors of survival in patients with resected pancreatic ductal adenocarcinoma. Dig Surg 2008;25:226-32. [PubMed]
- Peng YF, Yu WD, Pan HD, et al. Tumor regression grades: potential outcome predictor of locally advanced rectal adenocarcinoma after preoperative radiotherapy. World J Gastroenterol 2015;21:1851-6. [PubMed]
- Braun OM, Neumeister B, Popp W, et al. Histologic tumor regression grades in squamous cell carcinoma of the head and neck after preoperative radiochemotherapy. Cancer 1989;63:1097-100. [PubMed]
- Hermann RM, Krech R, Hartlapp J, et al. The value of qualitative regression grading as a prognostic factor for survival after preoperative radiochemotherapy in patients with advanced head and neck cancer. Strahlenther Onkol 2001;177:277-82. [PubMed]
- Bachmann R, Bachmann J, Hungbauer A, et al. Impact of response evaluation for resectable esophageal adenocarcinoma - a retrospective cohort study. Int J Surg 2014;12:1025-30. [PubMed]
- Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol 2013;3:262. [PubMed]
- Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer 2012;118:3182-90. [PubMed]
- Hirata T, Teshima T, Nishiyama K, et al. Histopathological effects of preoperative chemoradiotherapy for pancreatic cancer: an analysis for the impact of radiation and gemcitabine doses. Radiother Oncol 2015;114:122-7. [PubMed]
- Barugola G, Partelli S, Crippa S, et al. Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. Am J Surg 2012;203:132-9. [PubMed]
- Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005;47:141-6. [PubMed]
- Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant Chemotherapy for Adenocarcinoma of the Pancreas: Treament Variables and Survival Duration. Ann Surg Oncol 2001;8:123-32. [PubMed]
- Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2005;63:320-3. [PubMed]
- Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;58:1017-21. [PubMed]
- Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009;115:665-72. [PubMed]
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. [PubMed]
- Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012;118:268-77. [PubMed]
- Ishikawa O, Ohhigashi H, Sasaki Y, et al. The histopathological effect of preoperative irradiation in adenocarcinoma of the periampullary region. Nihon Gan Chiryo Gakkai Shi 1988;23:720-7. [PubMed]
- White RR, Xie HB, Gottfried MR, et al. Significance of histological response to preoperative chemoradiotherapy for pancreatic cancer. Ann Surg Oncol 2005;12:214-21. [PubMed]
- Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg 2005;242:684-92. [PubMed]
- Swisher SG, Hofstetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg 2005;241:810-7; discussion 817-20. [PubMed]
- Becker K, Reim D, Novotny A, et al. Proposal for a multifactorial prognostic score that accurately classifies 3 groups of gastric carcinoma patients with different outcomes after neoadjuvant chemotherapy and surgery. Ann Surg 2012;256:1002-7. [PubMed]
- Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992;127:1335-9. [PubMed]
- Washington MK, Berlin J, Branton PA, et al. Protocol for the examination of specimens from patients with carcinoma of the distal extrahepatic bile ducts. Arch Pathol Lab Med 2010;134:e8-13. [PubMed]
- Shimosato Y, Oboshi O, Baba K. Histological evaluation of effects of radiotherapy and chemotherapy for carcinomas. Jpn J Clin Oncol 1971;1:19-35.
- Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487-95. [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [PubMed]


