Implications of mismatch repair-deficient status on management of early stage colorectal cancer
Introduction
Colorectal cancer (CRC) is a common malignancy with more than one million new cases occurring each year (1) and is the second leading cause of cancer deaths in Western countries. Disease stage remains the strongest prognostic variable and is the key determinant of patient management. Although most cases of CRC develop through a CIN pathway, approximately 15% of cases are characterized by microsatellite instability (MSI), a molecular marker of defective DNA mismatch repair (dMMR). The frequency of MSI varies according to the tumor stage with highest rates in early stage cancers that decreases with progression to locoregional and distant metastases (2). In this review we describe the molecular aspects of the MMR system and discuss the implications of MMR-deficient status in the clinical management of patients with early stage CRC.
Phenotypic features and molecular origin of deficient MMR CRC
CRC patients with dMMR tumor have distinct clinical and pathologic features, such as proximal colon predominance, poor differentiation and mucinous histology, with increased numbers of tumor-infiltrating lymphocytes (Table 1) (3). Tumors with dMMR are more common among stage II, and are relatively uncommon among metastatic CRCs (4). MMR-deficiency can arise from two distinct molecular alterations. Lynch syndrome (LS) accounts for approximately 3-4% of all CRCs and one third of all dMMR/MSI-associated CRC. It is inherited autosomal dominant and is caused by inactivating germline mutations in MMR genes (5), including MLH1, MSH2, and more rarely MSH6 and PMS2 (6). Germline mutations in an MMR gene followed by a second hit to the wild-type copy is needed to produce LS, and can occur due to point mutation, loss of heterozygosity or methylation. Patients with LS develop tumors at early ages, often between 20 and 30 years old (compared to median age of 69 years in sporadic CRC) and have increased rates of synchronous CRCs. While cancers of the colon and rectum are most common among LS patients, these patients can also develop cancers of the uterine endometrium, stomach, ovary, urinary tract, small intestine and other sites (7). The estimated cumulative risks of CRC by age 70 years for LS patients is approximately 50% in case of MLH1 or MSH2 mutations, with endometrial cancer as the second most common malignancy in these patients (8). CRCs from LS patients are significantly less likely to carry BRAFV600E mutation (Table 1). Among dMMR/MSI CRCs, BRAFV600E mutation testing thus can be performed to distinguish LS cases from sporadic tumors (9). Patients with suspected hereditary CRC should be referred for genetic counseling, where the identification of germline mutations and evaluation/screening of family members can be appropriately addressed.
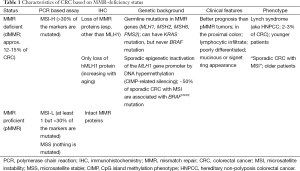
Full table
Among the 12-15% of all CRC tumors with dMMR/MSI, about two-thirds are sporadics. The majority of these cancers develop in a background of dense promoter hypermethylation of cancer-specific genes known as the CpG island methylator phenotype (CIMP) (10,11). CIMP-related silencing of the MLH1 gene is known to be responsible for about 80% of cases in which MLH1/PMS2 expression are lost (12). Approximately half of sporadic dMMR cases are associated with BRAFV600E mutations (13,14) that serve to distinguish them from LS cases (9). Patients with sporadic CRCs with MSI share clinicopathological features with LS cases with the exception that sporadics are substantially older at CRC diagnosis compared to LS and there is a female predominance (15).
Measuring MSI and dMMR
The DNA MMR system is composed of proteins (MLH1, MSH2, MSH3, MSH6, PMS2) whose function is to repair base-base mispairs introduced into short and tandemly-repeated sequences (microsatellites), during DNA synthesis to maintain genomic stability (3). Deficient MMR, due to genetic or epigenetic events, results in production of a truncated, nonfunctional protein or loss of a protein which causes MSI phenotype. Tumors with dMMR thus demonstrate a high frequency of MSI (MSI-H), and, in turn, MSI is used as the molecular fingerprint of dMMR.
MSI testing can be performed on paraffin-embedded tumor tissue using a PCR-based assay for detection of instability in selected microsatellite loci (16,17). A panel of microsatellite markers have been validated and recommended as a reference panel (18). On the basis of the MSI status, CRCs can be categorized into three groups: MSI-H, if 2 or more of the 5 microsatellite markers show instability (that is, have insertion/deletion mutations); MSI-L (low-frequency MSI), if only one of the five markers shows instability; and microsatellite stable (MSS) if none of the markers show instability (18). MSI-H corresponds to dMMR, whereas MSI-L and MSS indicate pMMR.
Analysis of MMR protein expression by immunohistochemistry (IHC) is an alternative test that is widely available with the advantages of not requiring a molecular laboratory and the ability to identify the affected gene by detecting loss of its protein product. Since the loss of MMR protein expression by IHC has been shown to be highly concordant with DNA-based MSI testing (17), these two tests are considered to be complimentary. Using IHC, tumors that demonstrate loss of an MMR protein can be collectively referred to as dMMR and expected to be MSI-H. Importantly, only loss of hMLH1 protein expression has been described in sporadic CRCs (12). Tumors with intact MMR proteins can be classified as proficient MMR that are MSS or MSI-low (MSI-L) (Table 1).
Mismatch repair (MMR)-deficient status and clinical outcome
Although the pathologic tumor stage remains the key determinant of CRC prognosis and treatment, there is considerable stage-independent variability in clinical outcome. Thus, new prognostic and predictive biomarkers are needed to inform prognosis and to guide the use and choice of systemic chemotherapy. Accumulating evidence indicates that dMMR status is one such candidate. Multiple studies have shown that patients with dMMR tumors have a more favorable stage-adjusted prognosis than those with pMMR tumors. These data are largely from retrospective studies that include clinical trials of adjuvant therapy (19-22), and a population-based study (23). A meta-analysis including 32 studies comprising 1,277 MSI cases, among a total of 7,642 patients with stage I-IV CRC, also showed a better prognosis for patients with dMMR compared with pMMR tumors (2). This analysis included untreated patients, as well as patients treated with 5-fluorouracil (5-FU)-based adjuvant chemotherapy. The hazard ratio (HR) for overall survival (OS) associated with dMMR was 0.65 [95% confidence interval (CI), 0.59-0.71]; the benefit was maintained when analyses were restricted to patients with stage II or stage III cancers participating in clinical studies (2). In general, the prognostic impact of dMMR appears to be stronger in earlier stage tumors, i.e., stage II versus node-positive or stage III cancers (24,25).
MMR status and 5-FU based adjuvant chemotherapy
The fluoropyrimidine 5-FU remains the most commonly used chemotherapy drug for the treatment of CRC. Where adjuvant chemotherapy remains optional in stage II CRC patients, capecitabine or 5-FU combined with leucovorin (LV), or combinations of these drugs with oxaliplatin, are considered to be standard treatment options for stage III. Preclinical models have suggested that dMMR tumors were associated with 5-FU resistance (26-31). The preponderance of evidence also suggests that 5-FU-based adjuvant chemotherapy is ineffective in patients with dMMR tumors (32), although some earlier studies suggested that patients with dMMR vs. pMMR tumors derive a similar or even a greater benefit from 5-FU-based adjuvant treatment (33-35). Conflicting results were based on studies where patients were not randomly assigned to 5-FU-based treatment versus observation after resection, a relatively small numbers of patients with dMMR colon cancers, and the bimodal age distribution among these patients. Accordingly, the impact of dMMR status as prognostic/predictive classifiers is ideally studied to a clinical trial cohort of same stage patients receiving uniform treatment.
Sargent et al. investigated 457 stage II and stage III colon cancer patients who were included in five randomized trials evaluating 5-FU + levamisole or LV as adjuvant chemotherapy vs. no post surgical treatment (36). In this analysis, patients with dMMR cancers had significantly better survival than did pMMR patients, although dMMR tumors of either stage did not benefit from 5-FU-based adjuvant therapy. These findings were validated by combining these data with those from a prior study by Ribic et al. from the same group (37), yielding a total of 1,027 stage II and stage III colon cancer patients. In the combined dataset, dMMR was associated with more favorable outcome compared to pMMR cancers (DFS: HR =0.51; 95% CI, 0.29-0.89; P=0.009; OS: HR =0.47; 95% CI, 0.26-0.83; P=0.004), and 5-FU adjuvant chemotherapy may attenuate the prognostic advantage of dMMR (DFS: HR =0.79; 95% CI, 0.4-1.25; P=0.30; OS: HR =0.78; 95% CI, 0.49-1.24; P=0.28). Of note, a suggestion of a detrimental effect of 5-FU was seen in patients with stage II dMMR tumors. These data were interpreted to indicate that patients with dMMR stage II CRC should not receive adjuvant 5-FU.
A lack of efficacy for 5-FU as adjuvant chemotherapy in patients with dMMR stage II CRC was observed in the Quick and Simple and Reliable (QUASAR) adjuvant therapy trial where patients with stage II CRCs were assigned to receive 5-FU (n=1,483) vs. surgery alone (n=1,480) (38). Among all patients with known MMR status, the risk of recurrence of dMMR tumors was reduced by half compared to pMMR tumors [11% (25 of 218) vs. 26% (438 of 1,695) recurred; risk ratio (RR) =0.53; 95% CI, 0.40-0.70; P<0.001]. However, MMR status did not predict benefit from chemotherapy (HR =0.97, P=0.92) (39). More recently, the prognostic impact of dMMR in stage II and III CRC patients was further examined using pooled data analysis from 17 adjuvant trials in the ACCENT database (40). This analysis involved 7,803 patients of which 571 received surgery alone and 3,878 patients received 5-FU monotherapy. Among stage II patients, dMMR vs. pMMR was strongly associated with increased TTR (HR =0.27; 95% CI, 0.10-0.75; P=0.01) and improved OS (HR =0.27; 95% CI, 0.10-0.74; P=0.01) in patients treated with surgery alone. However, such advantage of dMMR over pMMR was attenuated in patients treated with adjuvant 5-FU (TTR: HR =0.81, 95% CI, 0.55-1.19; P=0.29; OS: HR =0.87; 95% CI, 0.61-1.26; P=0.47). Among stage III patients receiving surgery alone, those with dMMR tumors were also found to have better outcome (TTR: HR =0.59; 95% CI, 0.28-1.23; P=0.16; OS: HR =0.69; 95% CI, 0.35-1.36; P=0.28) vs. pMMR cases. In stage III CRC patients, a significant survival benefit for 5-FU monotherapy vs. surgery alone was seen in patients with pMMR tumors (5-year TTR =64% vs. 47%), but also in patients with dMMR tumors (5-year TTR =72% vs. 60%). These findings support the current and recommended management of non-metastatic CRC whereby stage II patients with dMMR tumors are spared adjuvant 5-FU due to lack of efficacy, whereas all stage III patients received adjuvant chemotherapy irrespective of MMR status.
In a study that evaluated 2,141 stage II and stage III colon cancers from 5-FU-based adjuvant therapy trials, patients with dMMR colon cancers were shown to have reduced rates of tumor recurrence, delayed TTR, and improved survival rates compared with patient with pMMR cancers (41). Furthermore, an exploratory subset analysis suggested that dMMR tumors with suspected germline mutations (i.e., LS) had improved disease-free survival (DFS) after 5-FU-based treatment (DFS: HR =0.26; 95% CI, 0.09-0.77; P=0.009) compared with sporadic dMMR tumors where no benefit was observed (DFS: HR =0.79; 95% CI, 0.35-1.80; P=0.58). These preliminary findings raise the possibility that the utility of MMR status as a predictive factor for 5-FU treatment might differ according to the molecular mechanism underlying dMMR/MSI, which awaits further evaluation.
Treatment with standard 5-FU plus oxaliplatin adjuvant therapy
At present, the use of oxaliplatin in combination with adjuvant 5-FU chemotherapy is the standard of care for stage III colon cancer patients (42-44). Preclinical studies have shown that dMMR tumor cells are susceptible to oxaliplatin despite displaying resistance to 5-FU (45). To date, limited data are available for the prognostic/predictive impact of MMR on chemosensitivity to oxaliplatin-based treatment (46-49). In a retrospective study that included 303 unselected stage III colon cancer patients who received adjuvant FOLFOX, MMR status was a prognostic factor conferred a better DFS for patients with dMMR compared to pMMR tumors (50). Gavin et al. reported an analysis of 2,299 stage II and stage III colon cancers from participants in National Surgical Adjuvant Breast and Bowel Project (NSABP) adjuvant studies, including C-07 (5-FU plus LV ± oxaliplatin) and C-08 (FOLFOX ± bevacizumab) trials (51). The authors reported that dMMR was associated with better prognosis for recurrence in patients treated with FOLFOX compared with pMMR (TTR: HR =0.58; 95% CI, 0.35-0.96; P=0.03). However, MMR status was not predictive of oxaliplatin efficacy, since the interaction test between MMR status and treatment was not statistically significant. Flejou et al. reported the results of MMR status in 986 of the 2,240 patients enrolled in the Multicenter International Study of Oxaliplatin/5-FU LV in the Adjuvant Treatment of Colon Cancer (MOSAIC). The authors found that the DFS benefit from FOLFOX compared with 5-FU alone was also evident in patients with dMMR colon cancers (52). Taken together, available data suggest a potential benefit for oxaliplatin in node-positive dMMR colon cancers and therefore, do not support any change in the current therapy of these patients.
Patients treated with 5-FU plus irinotecan-based adjuvant therapy
It is important to emphasize that two randomized phase III studies [Cancer and Leukemia Group B (CALGB) 89803 (53) and Pan-European Trials in Alimentary Tract Cancers 3 (PETACC-3) trials (54)], failed to show the benefit of adding irinotecan to 5-FU as adjuvant chemotherapy in the treatment of stage III colon cancer patients. Thus unlike oxaliplatin, irinotecan is not used in the adjuvant setting. Preclinical studies including in vitro and xenograft model systems found that dMMR tumor cells exhibited sensitivity to irinotecan (55-57). In a retrospective analysis of 702 stage III colon cancer patients included in the CALGB 89803 trial, those with dMMR (n=96) who were treated with IFL (irinotecan, 5-FU and LV) had significantly improved 5-year DFS as compared with IFL-treated pMMR patients (n=606) (5-year DFS: 76% vs. 59%; HR =0.53; 95% CI, 0.29-0.96; log-rank P=0.03) (58) that was not observed among patients treated with 5-FU/LV. However, this finding was not supported by an analysis of 1,254 patients included in the PETACC-3 study (59) where the addition of irinotecan to 5-FU/LV did not show significantly improved survival in patients with dMMR tumors.
Patients treated with targeted therapies in an adjuvant setting
Recent success of biologic agents in the metastatic setting such as the use of antibodies directed against vascular endothelial growth factor (VEGF) or epidermal growth factor receptor (EGFR), resulted in the evaluation of these agents in the adjuvant setting. However, phase III adjuvant trials failed to show a significant survival benefit for anti-VEGF (60,61) or anti-EGFR antibodies (62-64) combined with adjuvant chemotherapy in patients with stage III colon cancer. In the NSABP C-08 trial where no benefit for the addition of bevacizumab to FOLFOX therapy was observed (60), a post hoc analysis found that patients with dMMR tumors derived a statistically significant survival benefit from the addition of bevacizumab (HR =0.52; 95% CI, 0.29-0.94; P=0.02) compared with patients with pMMR tumors (HR =1.03; 95% CI, 0.84-1.27; P=0.78) (65). The mechanism responsible for bevacizumab benefit in dMMR tumors is unknown, and confirmation of these data is needed. The North Central Cancer Treatment Group (NCCTG) N0147 trial tested the addition of cetuximab to FOLFOX (63) or FOLFIRI (irinotecan, 5-FU and LV) (64) adjuvant chemotherapy in the treatment of stage III colon cancer. The FOLFIRI-containing arms were discontinued (64) when other contemporary trials demonstrated no benefit to using irinotecan as adjuvant therapy (53,54). The addition of cetuximab to FOLFOX failed to improve DFS, the primary endpoint of this study compared to FOLFOX alone (63). Several biomarker analyses have been conducted using prospectively collected biospecimens from this study (25,66-69), where the treatment arms were combined based on the finding of no interaction between MMR status and treatment (FOLFOX ± cetuximab). In the N0147 trial, dMMR was detected in 314 (12%) of 2,580 stage III colon cancer patients and was not prognostic overall for DFS (HR =0.82; 95% CI, 0.64-1.07; 225 P=0.14) (66). Interestingly, favorable DFS was observed for dMMR vs. pMMR tumors in the proximal colon (HR =0.71; 95% CI, 0.53-0.94; P=0.018), but not in the distal colon (HR =1.71; 95% CI, 0.99-2.95; P=0.056), after adjustment for KRAS and BRAFV600E mutations and relevant covariates (66).
Conclusions
Abundant evidence suggests that MMR status is a valuable prognostic and predictive biomarker for non-metastatic CRC. Tumors with dMMR/MSI have a distinct phenotype and consistent data support dMMR as a biomarker of better stage-adjusted survival. While the majority of dMMR CRCs are sporadic, one-third arises in the setting of LS that has critical implications for patients and family members. To improve the identification of these patients in clinical practice, it has been recommended that all resected CRC be analyzed for MMR status. The excellent prognosis of resected stage II colon cancers with dMMR and evidence of lack of 5-FU benefit supports the recommendation to not administer adjuvant 5-FU chemotherapy in this population. In stage III CRC patients in whom oxaliplatin-based adjuvant chemotherapy is the current standard of care, there remain no convincing evidence to exclude such patients with dMMR tumors from receiving adjuvant FOLFOX. Accordingly, MMR status does not influence chemotherapy decisions in stage III patients. Recent and emerging data underscore molecular heterogeneity in CRCs and in the subset of dMMR tumors. Studies in pooled data from similar clinical trials may help to further explore this tumor heterogeneity and to decipher its impact on patient prognosis and on the efficacy of current chemotherapy regimens.
Acknowledgements
Funding: H Kawakami is supported by a fellowship grant from the Uehara Memorial Foundation. FA Sinicrope is supported by a National Cancer Institute Senior Scientist Award (Grant No. K05CA-142885).
Footnote
Conflicts of Interest: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609-18. [PubMed]
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073-87.e3.
- Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266-73. [PubMed]
- Boland CR. Evolution of the nomenclature for the hereditary colorectal cancer syndromes. Fam Cancer 2005;4:211-8. [PubMed]
- Peltomäki P. Lynch syndrome genes. Fam Cancer 2005;4:227-32. [PubMed]
- Watson P, Vasen HF, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer 2008;123:444-9. [PubMed]
- Bonadona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011;305:2304-10. [PubMed]
- Domingo E, Niessen RC, Oliveira C, et al. BRAF-V600E is not involved in the colorectal tumorigenesis of HNPCC in patients with functional MLH1 and MSH2 genes. Oncogene 2005;24:3995-8. [PubMed]
- Toyota M, Issa JP. CpG island methylator phenotypes in aging and cancer. Semin Cancer Biol 1999;9:349-57. [PubMed]
- Hughes LA, Khalid-de Bakker CA, Smits KM, et al. The CpG island methylator phenotype in colorectal cancer: progress and problems. Biochim Biophys Acta 2012;1825:77-85.
- Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A 1998;95:6870-5. [PubMed]
- Wang L, Cunningham JM, Winters JL, et al. BRAF mutations in colon cancer are not likely attributable to defective DNA mismatch repair. Cancer Res 2003;63:5209-12. [PubMed]
- Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med 2009;361:98-9. [PubMed]
- Miyakura Y, Sugano K, Konishi F, et al. Extensive methylation of hMLH1 promoter region predominates in proximal colon cancer with microsatellite instability. Gastroenterology 2001;121:1300-9. [PubMed]
- Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816-9. [PubMed]
- Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20:1043-8. [PubMed]
- Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248-57. [PubMed]
- Sinicrope FA, Rego RL, Halling KC, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology 2006;131:729-37. [PubMed]
- Gafà R, Maestri I, Matteuzzi M, et al. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer 2000;89:2025-37. [PubMed]
- Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst 1999;91:1295-303. [PubMed]
- Lanza G, Gafa R, Santini A, et al. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol 2006;24:2359-67. [PubMed]
- Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev 2001;10:917-23. [PubMed]
- Roth AD, Delorenzi M, Tejpar S, et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst 2012;104:1635-46. [PubMed]
- Sinicrope FA, Shi Q, Smyrk TC, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology 2015;148:88-99. [PubMed]
- Li LS, Morales JC, Veigl M, et al. DNA mismatch repair (MMR)-dependent 5-fluorouracil cytotoxicity and the potential for new therapeutic targets. Br J Pharmacol 2009;158:679-92. [PubMed]
- Davis TW, Wilson-Van Patten C, Meyers M, et al. Defective expression of the DNA mismatch repair protein, MLH1, alters G2-M cell cycle checkpoint arrest following ionizing radiation. Cancer Res 1998;58:767-78. [PubMed]
- Meyers M, Wagner MW, Hwang HS, et al. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res 2001;61:5193-201. [PubMed]
- Koi M, Umar A, Chauhan DP, et al. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N'-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res 1994;54:4308-12. [PubMed]
- Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer 2003;106:66-73. [PubMed]
- Fischer F, Baerenfaller K, Jiricny J. 5-Fluorouracil is efficiently removed from DNA by the base excision and mismatch repair systems. Gastroenterology 2007;133:1858-68. [PubMed]
- Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol 2010;7:153-62. [PubMed]
- Elsaleh H, Joseph D, Grieu F, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 2000;355:1745-50. [PubMed]
- Hemminki A, Mecklin JP, Jarvinen H, et al. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology 2000;119:921-8. [PubMed]
- Westra JL, Schaapveld M, Hollema H, et al. Determination of TP53 mutation is more relevant than microsatellite instability status for the prediction of disease-free survival in adjuvant-treated stage III colon cancer patients. J Clin Oncol 2005;23:5635-43. [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26. [PubMed]
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247-57. [PubMed]
- Quasar Collaborative Group1, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9.
- Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261-70. [PubMed]
- Sargent DJ, Shi Q, Yothers G, et al. Prognostic impact of deficient mismatch repair (dMMR) in 7,803 stage II/III colon cancer (CC) patients (pts): A pooled individual pt data analysis of 17 adjuvant trials in the ACCENT database. J Clin Oncol 2014;32:5s.
- Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst 2011;103:863-75. [PubMed]
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. [PubMed]
- Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. [PubMed]
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71. [PubMed]
- Fink D, Nebel S, Aebi S, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res 1996;56:4881-6. [PubMed]
- Des Guetz G, Lecaille C, Mariani P, et al. Prognostic impact of microsatellite instability in colorectal cancer patients treated with adjuvant FOLFOX. Anticancer Res 2010;30:4297-301. [PubMed]
- Zaanan A, Cuilliere-Dartigues P, Guilloux A, et al. Impact of p53 expression and microsatellite instability on stage III colon cancer disease-free survival in patients treated by 5-fluorouracil and leucovorin with or without oxaliplatin. Ann Oncol 2010;21:772-80. [PubMed]
- Kim ST, Lee J, Park SH, et al. Clinical impact of microsatellite instability in colon cancer following adjuvant FOLFOX therapy. Cancer Chemother Pharmacol 2010;66:659-67. [PubMed]
- Zaanan A, Meunier K, Sangar F, et al. Microsatellite instability in colorectal cancer: from molecular oncogenic mechanisms to clinical implications. Cell Oncol (Dordr) 2011;34:155-76. [PubMed]
- Zaanan A, Flejou JF, Emile JF, et al. Defective mismatch repair status as a prognostic biomarker of disease-free survival in stage III colon cancer patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer Res 2011;17:7470-8. [PubMed]
- Gavin PG, Paik S, Yothers G, et al. Colon cancer mutation: prognosis/prediction--response. Clin Cancer Res 2013;19:1301. [PubMed]
- Flejou JF, Andre T, Chibaudel B, et al. Effect of adding oxaliplatin to adjuvant 5-fluorouracil/leucovorin (5FU/LV) in patients with defective mismatch repair (dMMR) colon cancer stage II and III included in the MOSIAC study (abstract). J Clin Oncol 2013;31:abstr 3524.
- Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007;25:3456-61. [PubMed]
- Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117-25. [PubMed]
- Vilar E, Scaltriti M, Balmana J, et al. Microsatellite instability due to hMLH1 deficiency is associated with increased cytotoxicity to irinotecan in human colorectal cancer cell lines. Br J Cancer 2008;99:1607-12. [PubMed]
- Magrini R, Bhonde MR, Hanski ML, et al. Cellular effects of CPT-11 on colon carcinoma cells: dependence on p53 and hMLH1 status. Int J Cancer 2002;101:23-31. [PubMed]
- Bras-Gonçalves RA, Rosty C, Laurent-Puig P, et al. Sensitivity to CPT-11 of xenografted human colorectal cancers as a function of microsatellite instability and p53 status. Br J Cancer 2000;82:913-23. [PubMed]
- Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol 2009;27:1814-21. [PubMed]
- Klingbiel D, Saridaki Z, Roth AD, et al. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol 2015;26:126-32. [PubMed]
- Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 2011;29:11-6. [PubMed]
- de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33. [PubMed]
- Taieb J, Tabernero J, Mini E, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:862-73. [PubMed]
- Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383-93. [PubMed]
- Huang J, Nair SG, Mahoney MR, et al. Comparison of FOLFIRI with or without cetuximab in patients with resected stage iii colon cancer; NCCTG (Alliance) intergroup trial N0147. Clin Colorectal Cancer 2014;13:100-9. [PubMed]
- Pogue-Geile K, Yothers G, Taniyama Y, et al. Defective mismatch repair and benefit from bevacizumab for colon cancer: findings from NSABP C-08. J Natl Cancer Inst 2013;105:989-92. [PubMed]
- Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol 2013;31:3664-72. [PubMed]
- Gonsalves WI, Mahoney MR, Sargent DJ, et al. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst 2014.106. [PubMed]
- Sha D, Lee AM, Shi Q, et al. Association study of the let-7 miRNA-complementary site variant in the 3' untranslated region of the KRAS gene in stage III colon cancer (NCCTG N0147 Clinical Trial). Clin Cancer Res 2014;20:3319-27. [PubMed]
- Yoon HH, Tougeron D, Shi Q, et al. KRAS codon 12 and 13 mutations in relation to disease-free survival in BRAF-wild-type stage III colon cancers from an adjuvant chemotherapy trial (N0147 alliance). Clin Cancer Res 2014;20:3033-43. [PubMed]


