A case of peripheral T-cell lymphoma, not otherwise specified in a HCV and HTLV-II-positive patient, diagnosed by abdominal fluid cytology
Introduction
Peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS) is a highly aggressive, rare neoplasm that comprises about 25-29% of all PTCL, and represents over 15% of all lymphomas (1). It occurs mainly in adults and is more common in males than in females (2).
PTCL, NOS lymphoma is a heterogeneous group of nodal and extranodal mature T-cell lymphomas that do not match to any other defined categories of mature T-cell lymphoma in the current WHO classification. Peripheral lymph nodes are the most common site of involvement, but any site can be involved. Skin and gastrointestinal tract is the most common site of extranodal involvement (2). PTCL, NOS presenting as malignant ascites is extremely rare; only two cases of PTCL, NOS presenting with malignant ascites has been reported in the English literature so far (3,4). We are presenting a case of new onset malignant ascites from PTCL, NOS in a human T lymphotrophic virus II (HTLV-II) and hepatitis C positive patient.
Case presentation
A 61-year-old African-American male with past medical history of hepatitis C, cryoglobulinemia, membranoproliferative glomerulonephritis (MPGN), and treated cryptococcal pneumonia was admitted due to shortness of breath and worsening dyspnea on exertion over a period of 1 month. He had new onset abdominal distension that developed over 2 weeks prior to the hospitalization.
Physical examination was remarkable for distended abdomen with fluid wave, peripheral edema and bibasilar rales; although no palpable hepatomegaly or lymphadenopathy was noted.
Laboratory findings were remarkable for creatinine 1.30 mg/dL (baseline 0.8-0.9 mg/dL). Liver function tests included: ALT 28 U/L (normal range, 9-51 U/L), AST 71 U/L (normal range, 13-40 U/L) and alkaline phosphatase 108 U/L (normal range, 34-122 U/L). Cryptococcal antigen and HIV antibodies were negative. The patient was anemic with a hemoglobin of 9.4 g/dL, and RBC: 3.55×106/μL, WBC: 5.5×103/μL, platelet: 141×103/μL.
CT scan of the chest without contrast showed left pleural effusion and supradiaphragmatic, retroperitoneal, celiac and left axillary lymphadenopathies. Abdominal ultrasonography confirmed the presence of ascites and showed an enlarged liver with smooth contour and diffuse coarse echotexture. Spleen was also enlarged.
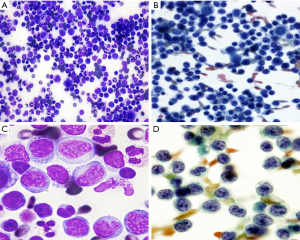
The patient underwent a paracentesis and 1.3 liter of abdominal fluid was removed. The examination of Cytospin and Thin Prep smears of the ascitic fluid revealed a very cellular smear consisting of a monomorphic population of intermediate-sized lymphoid cells with scant cytoplasm, irregular to convoluted nuclear contours; some morphologically mimic “flower cells” with multi-lobated nuclei (Figure 1). Flow cytometry on the abdominal fluid proved a T-cell lymphoproliferative disorder with atypical population expressing CD4, CD2, CD5 and weak surface CD3 but lack of CD7, CD16, CD56, CD57 and TdT (Figure 2). PCR studies detected a clonal T-cell population with T-cell receptor gamma gene rearrangement. Serological testing by EIA and Western Blot detected the antibody against HTLV-II. A subsequent bone marrow biopsy revealed the nodular and interstitial lymphomatous involvement. CD30 and ALK-1 were negative by immunohistochemical studies. Cytogenetics studies from the bone marrow aspirate showed normal male karyotype.

This case was classified as PTCL, NOS. Treatment of malignancy was not feasible due to the patient’s poor pulmonary function as well as financial constraints.
Discussion
PTCL are aggressive lymphomas that have poorer outcome relative to high-grade B cell lymphomas (5). In the International Peripheral T-cell Lymphoma Study, PTCL, NOS was the most frequent subtype of mature T-cell lymphomas, composing about 29.9% of mature PTCL (5). Generalized lymphadenopathy is the most common presentation of the PTCL, NOS but any site can be involved (2). PTCL, NOS presenting as malignant ascites is extremely uncommon.
Effusions involving peritoneal and pleural cavities during the course of hematological malignancies are not common (6). Malignant ascites, showing the presence of malignant cells in the peritoneal cavity, consist of approximately 10% of all cases of ascites (7). The development malignant ascites is a complex, multifactorial course involving a combination of impaired lymphatic drainage by tumor load and increased vascular permeability (8). Malignant ascites due to extensive infiltration of lymphomas is rare; and primary presentation of lymphomas with ascites is even rarer (9). According to the study of Karaosmanoglu et al. (10), among the malignant ascites due to lymphoma, diffuse large B cell lymphoma was the most common subtype of the lymphoma with peritoneal involvement while in their study, only one case of T-cell lymphoma with ascites was present. PTCL, NOS presenting as a malignant ascites is extremely rare. To the best of our knowledge, only two cases of PTCL, NOS presenting predominantly with ascites has been reported to date in the English literature (3,4).
The role of HCV in non-Hodgkin lymphomas (NHL) such as T-cell lymphomas is not clear. While numerous studies have confirmed an association between HCV infection and B-cell NHL (11-16); relatively few reports have addressed the role of HCV in T-cell lymphomas. In this case, patient was infected with HCV genotype 1.
Upon further workup of our case, serologic testing for HTLV was positive for HTLV-II. HTLV-I and HTLV-II are two distinct oncogenic retroviruses in humans. HTLV-1 infects CD4 positive T lymphocytes and is associated with adult T cell leukemia/lymphoma (17,18). HTLV-I is also associated with a chronic neurological disorder called myelopathy/tropical spastic paraparesis (HAM/TSP) (19). HTLV-I and -II share similar viral genome structures and both viruses encode transforming proteins called Tax (20,21). The role of HTLV-II for stimulation of leukemia is not clear; although the HTLV-II genome was detected in some cases of hairy cell leukemia (22). Also, Ren et al. suggested that Tax2 (HTLV-II Tax) can efficiently promote survival and aberrant proliferation of CD4+ T cells through several mechanisms (23). The significance of HTLV-II infection in this patient is unknown. To the best of our knowledge, there is no published reported association between HTLV-II and PTCL, NOS. However, the synergetic effects of both HTLV-II and HCV to the lymphoma genesis in this patient can’t be ruled out.
The common feature of the previous two reported cases (3,4) of PTCL, NOS with ascites and our case is the contribution of the cytological work-up of ascites for a rapid diagnosis. Ascites as the initial clinical manifestation of PTCL, NOS as in our case is extremely rare. Our case indicates that T-cell lymphoma should also be evaluated in the differential diagnosis when lymphoma is suspected in cytology fluid specimens.
In conclusion, PTCL, NOS can have unusual clinical presentation such as ascites and pleural effusion, and may also occur as a complication of immunodeficiency state. Further studies are needed to determine if HCV or HTLV-II viral infection is associated with PTCL, NOS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Savage KJ, Ferreri AJ, Zinzani PL, et al. Peripheral T-cell lymphoma--not otherwise specified. Crit Rev Oncol Hematol 2011;79:321-9. [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues, 4th ed. Lyon: IARC Press, 2008:285-8.
- Vakar-López F, Yang M. Peripheral T-cell lymphoma presenting as ascites: a case report and review of the literature. Diagn Cytopathol 1999;20:382-4. [PubMed]
- Izban KF, Pooley RJ Jr, Selvaggi SM, et al. Cytologic diagnosis of peripheral T-cell lymphoma manifesting as ascites. A case report. Acta Cytol 2001;45:385-92. [PubMed]
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124-30. [PubMed]
- Attanoos R. Lymphoproliferative conditions of the serosa. Arch Pathol Lab Med 2012;136:268-76. [PubMed]
- Malik I, Abubakar S, Rizwana I, et al. Clinical features and management of malignant ascites. J Pak Med Assoc 1991;41:38-40. [PubMed]
- Sangisetty SL, Miner TJ. Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg 2012;4:87-95. [PubMed]
- Kim Y, Cho O, Song S, et al. Peritoneal lymphomatosis: CT findings. Abdom Imaging 1998;23:87-90. [PubMed]
- Karaosmanoglu D, Karcaaltincaba M, Oguz B, et al. CT findings of lymphoma with peritoneal, omental and mesenteric involvement: peritoneal lymphomatosis. Eur J Radiol 2009;71:313-7. [PubMed]
- Ferri C, Caracciolo F, Zignego AL, et al. Hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Br J Haematol 1994;88:392-4. [PubMed]
- Pozzato G, Mazzaro C, Crovatto M, et al. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood 1994;84:3047-53. [PubMed]
- Mele A, Pulsoni A, Bianco E, et al. Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter case-control study. Blood 2003;102:996-9. [PubMed]
- Duberg AS, Nordström M, Törner A, et al. Non-Hodgkin’s lymphoma and other nonhepatic malignancies in Swedish patients with hepatitis C virus infection. Hepatology 2005;41:652-9. [PubMed]
- Anderson LA, Pfeiffer R, Warren JL, et al. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev 2008;17:3069-75. [PubMed]
- Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2006;15:2078-85. [PubMed]
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A 1980;77:7415-9. [PubMed]
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer 2007;7:270-80. [PubMed]
- Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet 1986;1:1031-2. [PubMed]
- Higuchi M, Fujii M. Distinct functions of HTLV-1 Tax1 from HTLV-2 Tax2 contribute key roles to viral pathogenesis. Retrovirology 2009;6:117. [PubMed]
- Feuer G, Green PL. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene 2005;24:5996-6004. [PubMed]
- Wang TG, Ye J, Lairmore MD, et al. In vitro cellular tropism of human T cell leukemia virus type 2. AIDS Res Hum Retroviruses 2000;16:1661-8. [PubMed]
- Ren T, Dong W, Takahashi Y, et al. HTLV-2 Tax immortalizes human CD4+ memory T lymphocytes by oncogenic activation and dysregulation of autophagy. J Biol Chem 2012;287:34683-93. [PubMed]


