Merkel cell carcinoma of unknown primary with lymph node and mesenteric metastasis involving the pancreas and duodenum
Introduction
Merkel cell carcinoma (MCC) is an unusual skin malignancy, also termed as neuroendocrine or primary small cell carcinoma of skin. It predominantly affects older Caucasians (1). However, it can occur in younger age in immunosuppressed individuals. There is an increased risk of MCC in patients with other malignancies (2,3). Ultraviolet radiation exposure plays an important role in its etiology (4,5). MCC has been described in patients treated with photochemotherapy (6). Merkel cell polyoma virus, a non-enveloped, dsDNA virus is linked to development of MCC (7). Face, upper limbs including shoulders are predominantly involved. It arises from Merkel cells at dermo-epidermal junction, which are of neuroendocrine origin. MCC typically presents with rapidly growing, firm, painless intracutaneous nodule (8). Histologically, it holds similarity to other poorly differentiated “small blue cell tumors”. Definitive diagnosis is established with immunohistochemical detection of intermediate filaments such as cytokeratins. MCC consistently stains positively for cytokeratin 20 (9,10). MCC has aggressive behavior with early and distant metastasis. The most common sites of metastasis are lymph nodes, distant skin, bone, and brain. In general, metastasis to the gastrointestinal tract is very uncommon. As few case reports are found in literature, we share our patient who presented with abdominal pain and constipation 1 year after being diagnosed with MCC in the lymph node with no known primary origin.
Case presentation
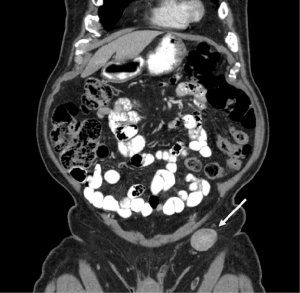
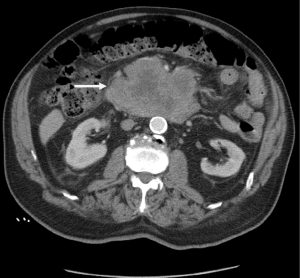
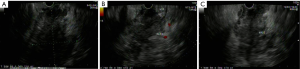
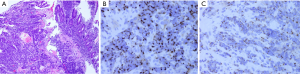
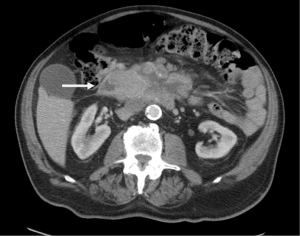
An 85-year-old Caucasian male presented to the emergency department with a three week history of worsening upper abdominal pain, abdominal distension, nausea, and constipation. He originally underwent an excisional biopsy of his left inguinal lymph node after a mass was observed on computerized tomography (CT) (Figure 1). A diagnosis of poorly differentiated neuroendocrine carcinoma, suggestive of MCC, was established. He completed radiation therapy (RT) of the left inguinal region. Physical examination revealed a solid epigastric mass without any tenderness. The rest of the physical exam was unremarkable. Initial labs were positive for sodium of 115 and hemoglobin of 11.9. FOBT was negative. Iron level, thyroid studies, lipase, and liver function tests (LFTs) were all within reference range. Serum osmolality was low. He reported a history of SIADH diagnosed 6 years ago and had been taking salt tablets. Patient admitted a recent reduction of salt tablets. CT abdomen/pelvis with contrast demonstrated a 10.2 cm × 6.3 cm partially necrotic mass in the mesentery of the mid abdomen immediately inferior to the head of the pancreas and anterior to the third portion of the duodenum (Figure 2). Dilated loops of intestine were present without any obstruction. CT guided biopsy of the mesenteric mass by interventional radiology was unsuccessful secondary to overlying duodenum. Subsequently, he was discharged home after correction of his serum sodium with outpatient endoscopic ultrasound (EUS) scheduled. EUS revealed a large heterogeneous mass (visualized with the ultrasound transducer in the duodenal bulb/apex) with hypoechoic, isoechoic, and anechoic areas. The mass had irregular borders and increased vascularity in the periphery. While the entire lesion could not be captured in one sonographic view, the largest dimensions measured 68.1 mm × 58 mm (Figure 3A). It was invading the head of pancreas and duodenal wall, growing through the muscularis propria layer of the duodenum (Figure 3B,C). Fine needle aspiration was performed and histological/immunologic profiles were consistent with MCC (Figure 4A-C). Chemotherapy with carboplatin and etoposide was initiated. After the second cycle of chemotherapy, he was hospitalized for hyponatremia and elevated transaminases. Repeat CT abdomen/pelvis revealed increased nodular masses at the root of the mesentery consistent with progression of disease (Figure 5). While hospitalized, patient had continued elevation in his LFTs and became jaundiced. Magnetic resonance cholangiopancreatography was performed demonstrating intra and extrahepatic ductal dilation with high grade obstruction at the distal common bile duct. Endoscopic retrograde cholangiopancreatography was attempted but not possible due to a large fungating tumor in the duodenum causing obstruction. The stricture was dilated with a balloon but the passage or duodenoscope was still not possible. A metal duodenal stent was placed. Thereafter, percutaneous transhepatic cholangiography was performed with successful placement of an internal/external biliary drainage catheter. Hospital course was complicated with sepsis secondary to bacteremia, acute renal failure, and gastrointestinal bleed. Patient and family eventually decided on hospice care considering the disease progression, poor prognosis, and the fact that additional chemotherapy with a second line chemo agent topotecan has no proven survival benefit.
Discussion
MCC is a rare skin cancer with high risk of metastasis and fatality (11). It was first described in 1972 by Toker. He referred to the first five cases of MCC as “trabecular carcinoma of the skin”, derived from the trabecular architecture of the tumor (12). Neuroendocrine or primary small cell carcinoma of the skin is the other synonyms used earlier. The term “Merkel cell carcinoma” was established in mid 1980s. The term “Primary neuroendocrine carcinoma of the skin” reflects the pathophysiology of the tumor.
MCC is typically seen in elderly Caucasians, the average age at presentation is about 75 years (1). Immunosuppression secondary to organ transplantation, B cell malignancies and HIV infection (2,13,14) could contribute to early occurrence of MCC, often diagnosed below age of 50. Females noted to have higher incidence than males. MCC has been associated with other skin tumors (squamous cell carcinoma, basal cell carcinoma) and hematological malignancies (B-cell, multiple myeloma) (2,3). Patients with MCC also exhibit increased risk for chronic lymphocytic leukemia.
MCC is believed to arise from Merkel cells that are located at the dermo-epidermal junction. Majority of MCCs are intradermal, only 10% arise in the epidermis (15). A German histopathologist, Friedrich Sigmund Merkel described “Merkel cell” for the first time in 1875, postulated that the cells act as mechanoreceptors in the skin (16). Pathogenetic factors such as UV irradiation, infection with Merkel cell polyoma virus and immunosuppression contribute to the tumor development. Incidence rates correlate with increasing sun exposure and a few cases have been reported in patients treated with PUVA (psoralen & ultraviolet A) photochemotherapy (4-6). Prevalence rates for Merkel cell polyoma virus in tumors of MCC vary, but most studies have found virus in 80% of MCC cases (7,17).
Patients with MCC typically present with rapidly growing, firm, painless, non-tender, shiny, flesh-colored or bluish-red, intracutaneous nodule (8). A population based study involving 3,778 cases of MCC has shown the highest incidence of MCC was observed in the skin of face (27%) followed by upper limbs and shoulder (22%), lower limbs and hip (15%), trunk (11%), scalp and neck (9%) (1). A high index of suspicion is required if the diagnosis is to be made without delay as the clinical presentation of this tumor is rather non-specific. In a large series study, 60-75% patients had localized disease at presentation while 10-25% had regional lymph node involvement; among them 14% without an identifiable primary tumor was noted. Although distant metastases are uncommon, one-third to one-half of patients with MCC eventually develop systemic disease. The most common sites involved are liver, lungs, bone, distant skin and lymph nodes (18,19).
MCC is a diagnostic challenge as the presentation does not always involve any typical features. Histological examination, strongly supported with immunohistochemistry is the basis for prompt diagnosis. MCC usually arises in dermis and extends into subcutis. Microscopically, MCC appears as “small blue cell tumors”, differential diagnosis include small cell carcinoma of lung, small B-cell lymphoma, Ewing sarcoma (20). The three main histologic patterns demonstrated are intermediate type (most common), trabecular type and small cell type. On immunohistochemistry, MCC has properties of both epithelial and neuroendocrine cells. Immunoreactivity for intermediate filaments such as cytokeratins distinguishes MCC from other undifferentiated tumors. MCC consistently stains positively for cytokeratin 20 (89-100% cases) and synaptophysin (9,10). Definitive diagnosis also requires negative reactivity for S100, leukocyte common antigen to differentiate from melanoma, lymphoma respectively (10).
MCC has a natural course which is unpredictable; disease is mostly assessed based on the stage at presentation. The cancer progression occurs rapidly, and the tumor may attain a large diameter in a short period of time. National Comprehensive Cancer Network (NCCN) provides a detailed therapeutic approach for patients with MCC (21). The initial management of the primary tumor is wide local excision of the primary MCC tumor with negative margins (22). MCC is a highly radiosensitive tumor, and RT can be used to as the only therapy in individuals who are not ideal surgical candidates. Lesions assessed to be at increased risk for local recurrence requires adjuvant RT at the resection site. Sentinel lymph node biopsy (SLNB) should be done in patients without any evidence of regional disease clinically (23). Regional lymph node dissection is commonly done with or without RT in positive nodal cases without any established diagnosis of distant metastasis.
In patients with asymptomatic primary MCC, nodal metastasis might be identified by performing SLNB. Like any other oncological detection, Positron emission tomography-computerized tomography (PET-CT) scan helps to locate distant metastasis. RT and chemotherapy (combination of etoposide and cisplatin) are the established approach of treatment in patients with advanced metastasis (22,24). Multidisciplinary approach is recommended in cases of distant metastasis.
The prognosis is rather poor. Stage of disease at presentation predicts overall survival (21). The presence of nodal disease is the most powerful predictor of survival and risk of developing distant metastatic disease. The site of primary lesion also impacts on prognosis. MCC arising in head & neck are difficult to treat. Leg lesions are highly associated with recurrence (25). Female sex, localized disease and younger age are positive predictors of survival. Local recurrences are very common, occurring in up to 44% of patients. The 5-year survival rate is between 30% and 64%. Mortality rate of MCC exceeds that of any other skin cancers (19). Frequent follow up is highly recommended in MCC patients secondary to notable incidence rates of recurrence, may be either local or disseminated. Follow up should be individualized according to risk factors and therapeutic options. Serum neuron-specific enolase has demonstrated utility for early detection of recurrence (26).
Conclusions
MCC is a rare, aggressive cutaneous malignancy and carries dismal prognosis. It is not often suspected as primary diagnosis because of its non-specific presentation. Histological and immunohistochemical profile play a significant role in diagnosis. MCC is sometimes diagnosed pathologically based upon lymph node involvement or other distant sites in the absence of an identifiable primary tumor similar to our case presentation. Management should be individualized based upon the specific pattern of disease presentation. Surgery is the standard therapy in localized disease; RT and chemotherapy play a vital role in advanced stages of disease. Multidisciplinary approach may benefit the patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 2010;37:20-7. [PubMed]
- Howard RA, Dores GM, Curtis RE, et al. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomarkers Prev 2006;15:1545-9. [PubMed]
- Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst 2010;102:793-801. [PubMed]
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol 2003;49:832-41. [PubMed]
- Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev 1999;8:153-8. [PubMed]
- Lunder EJ, Stern RS. Merkel-cell carcinomas in patients treated with methoxsalen and ultraviolet A radiation. N Engl J Med 1998;339:1247-8. [PubMed]
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096-100. [PubMed]
- Becker JC. Merkel cell carcinoma. Ann Oncol 2010;21 Suppl 7:vii81-5. [PubMed]
- Warner TF, Uno H, Hafez GR, et al. Merkel cells and Merkel cell tumors. Ultrastructure, immunocytochemistry and review of the literature. Cancer 1983;52:238-45. [PubMed]
- Chan JK, Suster S, Wenig BM, et al. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol 1997;21:226-34. [PubMed]
- Houben R, Schrama D, Becker JC. Molecular pathogenesis of Merkel cell carcinoma. Exp Dermatol 2009;18:193-8. [PubMed]
- Toker C. Trabecular carcinoma of the skin. Arch Dermatol 1972;105:107-10. [PubMed]
- Penn I, First MR. Merkel's cell carcinoma in organ recipients: report of 41 cases. Transplantation 1999;68:1717-21. [PubMed]
- Manganoni MA, Farisoglio C, Tucci G, et al. Merkel cell carcinoma and HIV infection: a case report and review of the literature. AIDS Patient Care STDS 2007;21:447-51. [PubMed]
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol 1993;29:143-56. [PubMed]
- Merkel F. Tastzellen und Tastkörperchen bei den Haustieren und beim Menschen. Arkiv für Mikroskopische Anatomie und Entwicklungsmechanik 1875;11:636-52.
- Kassem A, Schöpflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res 2008;68:5009-13. [PubMed]
- Zhan FQ, Packianathan VS, Zeitouni NC. Merkel cell carcinoma: a review of current advances. J Natl Compr Canc Netw 2009;7:333-9. [PubMed]
- Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol 2005;23:2300-9. [PubMed]
- Smith DF, Messina JL, Perrott R, et al. Clinical approach to neuroendocrine carcinoma of the skin (Merkel cell carcinoma). Cancer Control 2000;7:72-83. [PubMed]
- NCCN Guidelines version 1.2014. Merkel Cell Carcinoma. Available online: http://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf
- Tai P. A practical update of surgical management of merkel cell carcinoma of the skin. ISRN Surg 2013;2013:850797.
- Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol 2011;29:1036-41. [PubMed]
- Siva S, Byrne K, Seel M, et al. 18F-FDG PET provides high-impact and powerful prognostic stratification in the staging of Merkel cell carcinoma: a 15-year institutional experience. J Nucl Med 2013;54:1223-9. [PubMed]
- Poulsen M. Merkel-cell carcinoma of the skin. Lancet Oncol 2004;5:593-9. [PubMed]
- Tai PT, Yu E, Tonita J, et al. Merkel cell carcinoma of the skin. J Cutan Med Surg 2000;4:186-95. [PubMed]


