Comparison of anal cancer outcomes in public and private hospital patients treated at a single radiation oncology center
Introduction
Despite significant advances in the diagnosis and treatment of the disease, cancer remains the 4th leading cause of death in adults in the United States. Socioeconomic status (SES) has been demonstrated to be associated with both cancer incidence and prognosis, with patients of low SES bearing a disproportionately large portion of the public health burden of the disease (1-3). However, the specific drivers of poorer outcome have not been fully elucidated, impeding our ability to act on these inequalities (4). In fact, while our understanding of cancer biology, screening techniques, and treatment has improved significantly over the past 20 years, SES disparities in cancer mortality have remained constant (5).
Anal cancer is an uncommon malignancy, with 7,210 new cases and 950 deaths yearly (6). Locally advanced anal cancer treated with chemotherapy and radiation has a survival outcome approaching 80%. Although there is a clear association between HPV infection and the development of anal cancer, molecular and biologic outcome predictors for survival are not well understood. Furthermore, because of its association with a transmittable virus, the incidence of this disease is rising worldwide (7-9). Most research into factors associated with anal cancer incidence and prognosis focuses on pathologic features of the disease (10-12). Since the largest gaps in survival based on SES has been found among the most treatable cancers, identifying and minimizing SES disparities in diagnosis and treatment among anal cancer patients is particularly valuable (1).
Many SES studies have used large national databases of multi-institutional trial data to assess disparities in cancer outcomes (13,14). One potential limitation of this approach is the confounding factor of variable treatment patterns between institutions. This study compares patients referred from either a private, not-for-profit hospital or a public safety net hospital to a single clinical cancer center, with hospital type serving as a surrogate for SES. Our public hospital primarily comprises uninsured or Medicaid-insured patients, many of whom are of racial and ethnic minorities, and/or do not speak English as a first language (15). We compared anal cancer clinical and treatment characteristics between patients referred from these two hospitals to a single cancer center where they received radiation therapy (RT) from the same treatment staff.
Methods
Study sites and patient inclusion criteria
We conducted a chart review on all patients from a private and public hospital who received RT anal cancer at the same clinical cancer center. Both facilities are located in midtown Manhattan. The private hospital is an integrated, not-for-profit academic medical center. The public hospital is an urban safety net hospital that largely serves underinsured patients. Thirty-one percent of clinic patients at this institution are uninsured and 45% are insured through Medicaid (15). In the primary service area of the public hospital, 57% of residents do not speak English as a first language, and 22% of families live below Federal poverty guidelines (15). The clinical cancer center is based at the private hospital, but is affiliated with the public hospital and provides RT for patients referred from both institutions. The NYU Department of Radiation Oncology routinely treats all patients referred from both institutions at the clinical cancer center. Thus, all patients in this review were treated with a unified protocol administered the by the NYU Radiation Oncology faculty, residents, and staff.
Between December 2004 and December 2013, 112 patients with locally advanced anal cancer were treated definitively with chemoradiation (CRT). Patients were excluded if CRT was delivered for patients with stage IV disease, recurrent disease, or if histology revealed anything but an epithelial cancer of anal canal. Three patients from the public hospital were excluded due to insufficient treatment data. We performed a retrospective analysis of the remaining 109 patients, with institutional review board approval.
Diagnosis and treatment
Patients were clinically staged with CT imaging. External beam RT was delivered as either 3-dimensional radiation therapy (3D RT) or intensity modulated radiation therapy (IMRT) with continuous standard fractionation. A minimum dose of 30.6 Gy was delivered to electively treated lymph nodes and a minimum dose of 45 Gy was delivered to the gross tumor. RT was delivered in 1.8 Gy fractions for all patients. Most patients also received continuous infusion of 5-fluorouracil 1,000 mg/m2/d IV on days 1-4 and 29-32 of treatment, and mitomycin C 10 mg/m2 IV bolus on days 1 and 29 of treatment.
Data
Data were collected on patient age at diagnosis, gender, race, insurance, HIV status, histology, and clinical stage at presentation. Date of pathologic diagnosis, dates of RT, RT dose, presence of unplanned RT breaks, chemotherapy regimens, and RT toxicities were recorded. Radiation toxicities were graded according to the Radiation Therapy and Oncology Group (RTOG) common toxicity criteria.
Statistical analysis
Outcome measures were tumor size stage at presentation, RT delay, RT duration, unplanned treatment breaks greater than or equal to 10 days, overall survival (OS), disease free survival (DFS) rate, and colostomy free survival (CFS). RT delay was defined as the interval from date of pathologic diagnosis to the first day of RT. OS was defined as time from initiation of CRT to death due to any cause or most recent follow-up. DFS was defined as time from initiation of CRT to the occurrence of local, regional, or distant recurrence, death, or most recent follow-up. CFS was measured from initiation of CRT to diverting colostomy or salvage abdominoperineal resection (APR), death, or most recent follow-up without surgery.
The associations between referral hospital and outcomes were examined using Student’s t-test to compare means and χ2 test to compare frequency, as appropriate. Survival curves for OS, DFS, and CFS for private hospital and public hospital were created using the Kaplan-Meier method and compared with the log-rank test. Confidence intervals (CIs) were calculated using the formula “95% CI = mean ± standard error ×1.96”. Unadjusted and adjusted hazard ratios for referral hospital, insurance status, and race were calculated using the Cox proportional hazards model. All statistical analyses were carried out using SPSS version 20 (IBM Corp., Armonk, NY, USA). Statistical tests were two-sided and P values <0.05 were considered significant.
Results
Patient population and characteristics
In our cohort, the charts of 109 patients undergoing CRT for anal cancer at the public and private hospital were reviewed. Seventy-seven patients were from the private hospital and 32 patients were from the public hospital. Demographic and clinical variables of the patients are reported in Table 1. The mean age of patients overall was 59.5 years (range, 24.4-93.2 years), with no significant difference between private hospital and public hospital patients (P=0.222). A total of 60.6% of patients were male, and the gender distribution was the same between the private and public hospital (P=0.554).
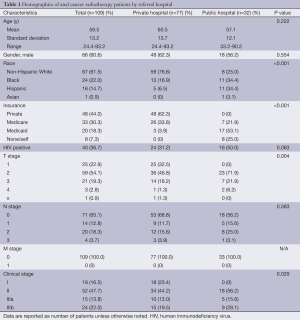
Full table
The majority of private hospital patients were non-Hispanic White (76.6%), while the majority of public hospital patients were Black (34.4%) or Hispanic (34.4%) (P<0.001). There were more patients with private insurance and Medicare at the private hospital, and more with Medicaid or uninsured at the public hospital (P<0.001). There were more HIV positive patients in the public hospital group compared to the private hospital group (50% vs. 31.2%, P=0.063, respectively).
Of 109 patients, 105 (96.3%) were found to have squamous cell carcinoma, one patient from each hospital had cloacogenic carcinoma, and one patient from each hospital had adenocarcinoma. Patients from the public hospital presented with higher T stage and AJCC stage group (P=0.004 and 0.029, respectively). There was no difference in N stage between the groups.
Treatment
The distribution of RT treatment course characteristics is described in Table 2. Most patients received 3D RT prior to 2009, and IMRT after 2009. There was no significant difference in the rate of IMRT, dose to electively treated lymph nodes, or dose to gross tumor. RT course was truncated in two patients from the private hospital and one from the public hospital. Concurrent chemotherapy was given in 99 patients (97.1%), with similar rates between the two hospitals. Eight patients received 5-fluorouracil only; 18 patients received alternative chemotherapy in addition to 5-fluorouracil, most commonly capecitabine (n=8) and cisplatin (n=5).
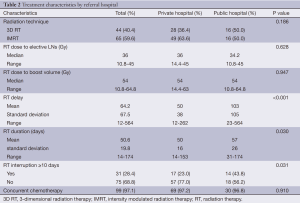
Full table
Public hospital patients had longer RT delay (103±105 vs. 50±38 days, P<0.001), and experienced significantly longer RT duration (57±26 vs. 50±16 days, P=0.03). More patients from the public hospital had a treatment interruption greater than or equal to 10 days (43.8% vs. 23%, P=0.031, respectively). When stratified by RT technique, there was no difference between the hospitals in RT duration (P=0.440) or presence of treatment breaks (P=0.655) among patients receiving 3D RT. Among patients receiving IMRT, public hospital patients experienced longer RT duration (62.8±8.3 vs. 43.0±33.6 days, P<0.001) and were more likely to have a break of 10 days or greater (50% vs. 10.6%, P=0.001). The duration of RT delay was not associated with the presence of unplanned treatment breaks (P=0.439).
Toxicity
The incidence of grade 3-4 dermatitis was greater in the public hospital group, while the incidence of fatigue and diarrhea was similar between the two hospitals (Table 3). There was no difference between the hospitals in the percentage of patients requiring growth factor support (P=0.646) or platelet or red blood cell transfusion (P=0.556) during CRT. There was no difference between the private and public hospitals in absolute neutrophil count nadir (1.8±1.0 vs. 1.7±1.1, P=0.726), white blood cell nadir (2.8±1.5 vs. 2.5±1.5, P=0.390), platelet nadir (98.5±56.9 vs. 128.3±99.4, P=0.154), or hemoglobin nadir (10.1±1.9 vs. 15.3±31.2, P=0.395).
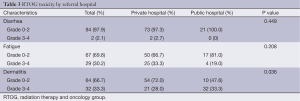
Full table
Patients with a treatment break of greater than or equal to 10 days were more likely to have grade 3-4 dermatitis toxicity (52.2% vs. 27.1%, P=0.027). There was no statistically significant correlation between treatment break and fatigue, diarrhea, or hematologic toxicity.
Clinical follow-up
Following CRT, patients were followed with palpation of the inguinal lymph nodes, digital rectal exam, and anoscopy every 3-6 months for 5 years, and with chest, abdominal, and pelvic imaging annually for 3 years. The median duration of follow-up was 14.9 mos (range, 0.7-94.8 mos), with no difference in follow-up time between the hospitals (P=0.150). At the time of this review, 80 patients (73.4%) were alive and 24 patients (22.0%) were dead. Five patients were lost to follow-up.
Patient outcomes
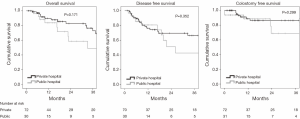
The Kaplan-Meier curves for OS, DFS, and CFS are presented in Figure 1. The three-year OS was 72.8% (95% CI, 63.4% to 76.6%) for private hospital patients and 48.9% (95% CI, 22.8% to 75.0%) for public hospital patients, hazard ratio (HR) 1.77 (95% CI, 0.77-4.07). The 3-year DFS was 66.3% (95% CI, 53.0% to 70.1%) for private hospital patents and 42.7% (95% CI, 16.6% to 68.8%) for public hospital patients, HR 1.41 (95% CI, 0.68-2.94). The three-year CFS was 86.4% (95% CI, 76.8% to 90.2%) for private hospital patients and 68.9% (42.8% to 95.0%) for public hospital patients, HR 1.82 (95% CI, 0.58-5.74).
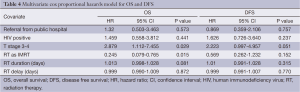
Multivariate analysis showed that referral from the public hospital did not confer a statistically significant increased risk of death or recurrence when potential confounders were controlled for (Table 4).
Full table
Discussion
In the study presented here, public hospital patients presented at more advanced stages and were more likely to have delayed RT, increased RT duration due to unplanned treatment interruptions, and treatment breaks greater than or equal to 10 days. RT courses were otherwise comparable between the two populations, as the patients received care at the same RT center. While the private hospital patients’ survival was similar to nationally and internationally reported statistics, OS and DFS was substantially lower for public hospital patients (7,16). It is critical to note that the patient size is small and the Kaplan-Meier curves cross, limiting the interpretation of the survival analysis in this retrospective review. However, a decreased survival is consistent with poorer outcomes associated with the observed differences in presentation and RT course.
On review of the literature, few studies have examined the effect of SES on anal cancer survival as a primary objective. One study analyzing the National Cancer Data Base (NCDB) found that income <$36,000 and Black and Hispanic race were associated with increased risk of death from anal cancer (17). An analysis of the Surveillance, Epidemiology, and End Results Program found poorer survival in Black patients between 1994 and 2000 (8). However, a more recent analysis of the NCDB between 2004 and 2014 found that race was not predictive of salvage APR (18). Our study is the first to compare differences in clinical presentation and treatment course between two different SES populations, allowing for the identification of potentially reversible inequalities in treatment characteristics that may contribute to poorer survival in low SES patients.
In the United States, anal cancer incidence is higher in females, however in this study the majority of patients were male (6). This discrepancy may reflect an increased prevalence of genital/anal HPV infections and sexual practices in our urban population, particularly given the high rate of HIV infection in our study. Of note, since the introduction of HAART, the incidence of anal cancer has been rising, particularly among men who have sex with men and those who are HIV-positive (9,17,19). Our study population may be reflective of this demographic shift, and increasing education and screening in this population may be appropriate.
A strength of our study is that all patients were treated at the same RT center, and hence RT treatment quality was the same despite differences in patterns of care. This allowed us to evaluate the effect of RT quality on SES disparities. It is well documented that patients treated at safety net hospitals and those insured through Medicaid are less likely to receive standard of care treatment (20,21). Particularly in the case of rare cancers, access to standard treatment provided by high volume, centralized cancer centers such as the one in this study is an important determinant of outcome (22,23). At this RT center, all patients are treated by the same staff in the same facility and are discussed at multidisciplinary tumor boards. Access to quality RT and careful coordination of care should mitigate differences in outcome between the two hospitals, but other factors come into play. More intensive care coordination and patient navigation may be required in low SES populations to improve survival.
The T-stage differential between the two hospital populations is the most apparent reason for the trends in DFS and OS favoring the private hospital patients. Advanced local stage at presentation is a known risk factor in anal cancer, and was associated with increased risk of death and disease recurrence in this study. More advanced presentation in low SES patients has been observed in other malignancies (5,24). These findings may be due to logistic, financial, language, cultural, and health literacy barriers to screening and care among low SES populations (25,26). Since anal cancer is generally symptomatic even at early stages, increasing community awareness of the warning signs for anal cancer may be a particularly effective way to improve anal cancer outcomes in low SES populations.
Multiple studies have reported longer delays to RT initiation in racial minorities in prostate, breast, and cervical cancer (27-29). These findings are consistent with the delay in RT observed in the public hospital patients. In addition to the barriers mentioned above, one explanation for this finding is that public hospitals are resource-limited, and therefore tend to have longer waits to obtain the necessary clinical appointments and imaging studies prior to treatment. In breast and head and neck cancer, longer time from diagnosis to RT initiation has been associated with increased risk of local recurrence and poorer survival (30-32). To the best of our knowledge, the prognostic significance of treatment delays has not previously been examined in anal cancer. Further investigation into its effect on outcome is warranted to clarify its role in anal cancer outcome disparities.
The etiology of the increased incidence of unplanned RT breaks in the public hospital population is likely multifactorial. First, public hospital patients were more likely to experience severe dermatitis, which is likely explained in part by the higher proportion of HIV-positive individuals in this group (33). Further, patients from racial and ethnic minorities report poorer physician information-sharing than white patients, potentially contributing to decreased compliance with RT (34). Additionally, navigating cancer treatment is difficult for all patients, and may be especially challenging for public hospital patients who face financial, language, and health literacy barriers (15). In 2010, Ben-Josef et al. analyzed data on 644 anal cancer patients from the RTOG 87-04 and RTOG 98-11 trials, and found that total treatment time, but not RT duration, negatively impacted local control and colostomy-free rate (11). While several small, retrospective studies have also demonstrated a trend toward poorer survival with RT interruption (35,36), others have not (37,38). Though the prognostic significance of RT breaks requires further study, low SES patients appear to be particularly vulnerable to treatment interruptions, and increasing compliance may improve outcomes in this population.
This study has several limitations that should be considered when interpreting the data. We were unable to collect information on income and education status, and therefore may have overlooked subsets of the low SES population that experienced poorer outcomes. We also did not have data on reasons for RT delay and RT interruptions, limiting our ability to identify specific drivers of treatment disparities. Additionally, it has been postulated that higher rates of medical comorbidities among low SES patients may contribute to poorer outcomes (4). Low SES populations, and in particular those treated at safety net hospitals, have significantly more comorbidities than the average population (39). It is possible that higher comorbidities in the public hospital patients may have contributed to RT delay and RT breaks, as well as differences in survival. Finally, the median follow-up time of 14.9 months is suboptimal. Historically, it has been difficult to achieve long-term follow-up in the public hospital population, as these patients have increased socioeconomic stressors that make long-term follow-up challenging. Finally, the small sample size limits our power to detect differences in survival. Nevertheless, the results are suggestive of a clinically meaningful difference and justify further study.
Conclusions
Despite receiving the same high-quality RT as the private hospital patients, the public hospital patients had poorer survival than expected in this study. We identified discrepancies in stage at diagnosis, treatment initiation, and compliance during RT between the two populations, suggesting that addressing these disparities may improve survival in low SES populations. Resources should be directed towards interventions that address these inequities, and outcomes should be studied prospectively. In the future, larger studies with longer follow-up may be needed to better understand the impact of SES on anal cancer survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kogevinas M, Porta M. Socioeconomic differences in cancer survival: a review of the evidence. IARC Sci Publ 1997;177-206. [PubMed]
- Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212-36. [PubMed]
- Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004;54:78-93. [PubMed]
- Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 2006;17:5-19. [PubMed]
- Aizer AA, Wilhite TJ, Chen MH, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer 2014;120:1532-9. [PubMed]
- Olivatto LO, Araujo CM, Vilhena B, et al. Phase I study of cetuximab (CET) in combination with 5-FU, cisplatin (CP), and radiotherapy (RT) in patients with locally advanced squamous cell anal canal carcinoma (LASACC). 2010 Gastrointestinal Cancers Symposium. Orlando, 2010.
- Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 2012;30:4344-51. [PubMed]
- Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer 2004;101:281-8. [PubMed]
- van der Zee RP, Richel O, de Vries HJ, et al. The increasing incidence of anal cancer: can it be explained by trends in risk groups? Neth J Med 2013;71:401-11. [PubMed]
- Glynne-Jones R, Sebag-Montefiore D, Adams R, et al. Prognostic factors for recurrence and survival in anal cancer: generating hypotheses from the mature outcomes of the first United Kingdom Coordinating Committee on Cancer Research Anal Cancer Trial (ACT I). Cancer 2013;119:748-55. [PubMed]
- Ben-Josef E, Moughan J, Ajani JA, et al. Impact of overall treatment time on survival and local control in patients with anal cancer: a pooled data analysis of Radiation Therapy Oncology Group trials 87-04 and 98-11. J Clin Oncol 2010;28:5061-6. [PubMed]
- Osti MF, Agolli L, Scaringi C, et al. Curative radiotherapy in patients with anal cancer: clinical outcomes and prognostic factors in a single-institution experience. Radiol Med 2013;118:882-94. [PubMed]
- Du KL, Bae K, Movsas B, et al. Impact of marital status and race on outcomes of patients enrolled in Radiation Therapy Oncology Group prostate cancer trials. Support Care Cancer 2012;20:1317-25. [PubMed]
- Konski A, Berkey BA, Kian Ang K, et al. Effect of education level on outcome of patients treated on Radiation Therapy Oncology Group Protocol 90-03. Cancer 2003;98:1497-503. [PubMed]
- Pressman M, Bohlen S, editors. 2013 Community Health Needs Assessment and Implementation Strategy. New York: Bellevue Hospital Center, 2013.
- James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol 2013;14:516-24. [PubMed]
- Bilimoria KY, Bentrem DJ, Rock CE, et al. Outcomes and prognostic factors for squamous-cell carcinoma of the anal canal: analysis of patients from the National Cancer Data Base. Dis Colon Rectum 2009;52:624-31. [PubMed]
- Geltzeiler CB, Nabavizadeh N, Kim J, et al. Chemoradiotherapy with a radiation boost for anal cancer decreases the risk for salvage abdominoperineal resection: analysis from the national cancer data base. Ann Surg Oncol 2014;21:3616-20. [PubMed]
- Chiao EY, Krown SE, Stier EA, et al. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr 2005;40:451-5. [PubMed]
- Harlan LC, Greene AL, Clegg LX, et al. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol 2005;23:9079-88. [PubMed]
- Werner RM, Goldman LE, Dudley RA. Comparison of change in quality of care between safety-net and non-safety-net hospitals. JAMA 2008;299:2180-7. [PubMed]
- Lin JF, Berger JL, Krivak TC, et al. Impact of facility volume on therapy and survival for locally advanced cervical cancer. Gynecol Oncol 2014;132:416-22. [PubMed]
- Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol 2000;18:2327-40. [PubMed]
- Lyratzopoulos G, Abel GA, Brown CH, et al. Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol 2013;24:843-50. [PubMed]
- Forbes LJ, Warburton F, Richards MA, et al. Risk factors for delay in symptomatic presentation: a survey of cancer patients. Br J Cancer 2014;111:581-8. [PubMed]
- Katz ML, Young GS, Reiter PL, et al. Barriers reported among patients with breast and cervical abnormalities in the patient navigation research program: impact on timely care. Womens Health Issues 2014;24:e155-62. [PubMed]
- Stokes WA, Hendrix LH, Royce TJ, et al. Racial differences in time from prostate cancer diagnosis to treatment initiation: a population-based study. Cancer 2013;119:2486-93. [PubMed]
- Gold HT, Thwin SS, Buist DS, et al. Delayed radiotherapy for breast cancer patients in integrated delivery systems. Am J Manag Care 2009;15:785-9. [PubMed]
- Ashing-Giwa K, Rosales M. Evaluation of therapeutic care delay among Latina- and European-American cervical cancer survivors. Gynecol Oncol 2013;128:160-5. [PubMed]
- Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol 2003;21:555-63. [PubMed]
- Chen Z, King W, Pearcey R, et al. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol 2008;87:3-16. [PubMed]
- Gold HT, Do HT, Dick AW. Correlates and effect of suboptimal radiotherapy in women with ductal carcinoma in situ or early invasive breast cancer. Cancer 2008;113:3108-15. [PubMed]
- Oehler-Jänne C, Huguet F, Provencher S, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol 2008;26:2550-7. [PubMed]
- Gordon HS, Street RL Jr, Sharf BF, et al. Racial differences in doctors' information-giving and patients' participation. Cancer 2006;107:1313-20. [PubMed]
- Weber DC, Kurtz JM, Allal AS. The impact of gap duration on local control in anal canal carcinoma treated by split-course radiotherapy and concomitant chemotherapy. Int J Radiat Oncol Biol Phys 2001;50:675-80. [PubMed]
- Konski A, Garcia M Jr, John M, et al. Evaluation of planned treatment breaks during radiation therapy for anal cancer: update of RTOG 92-08. Int J Radiat Oncol Biol Phys 2008;72:114-8. [PubMed]
- Janssen S, Meier zu Eissen J, Kolbert G, et al. Anal cancer treated with radio-chemotherapy: correlation between length of treatment interruption and outcome. Int J Colorectal Dis 2009;24:1421-8. [PubMed]
- Meyer A, Meier Zu Eissen J, Karstens JH, et al. Chemoradiotherapy in patients with anal cancer: impact of length of unplanned treatment interruption on outcome. Acta Oncol 2006;45:728-35. [PubMed]
- Genther DJ, Gourin CG. The effect of hospital safety-net burden status on short-term outcomes and cost of care after head and neck cancer surgery. Arch Otolaryngol Head Neck Surg 2012;138:1015-22. [PubMed]


