Surgery for gallbladder cancer in the US: a need for greater lymph node clearance
Introduction
Gallbladder cancer (GBC) is a rare but highly aggressive malignancy affecting approximately 9,810 new cases in 2012 (1). Since the advent of minimally invasive surgery, more cases are diagnosed incidentally after laparoscopic cholecystectomy for presume cholelithiasis or cholecystitis (2). The prognosis is often perceived with pessimism and nihilism given the lack of effective systemic therapy and the advanced stage with which it usually presents.
GBC is a rare and rapidly fatal disease due to its propensity for early dissemination. As a result, there have been few studies on the epidemiology of GBC. Women are three times more likely to develop GBC while significant variation is seen in the geographic distribution and prevalence of GBC (3,4). Although poorly understood, risk factors for the development of GBC include cholelithiasis, multiparity, obesity, history of typhoid infection, exposure of thoratrast, and porcelain gallbladder (3-5). A large international multi-institutional case-control study demonstrated that a history of gallbladder symptoms requiring medical attention was a major risk factor associated with GBC (6).
Surgery is widely regarded as the best treatment modality for non-metastatic GBC (7). Glenn and Jays first proposed radical cholecystectomy with lymphadenectomy for malignant extrahepatic biliary tract tumors in 1954 (8). Later, Pack et al. advocated for a more aggressive approach consisting of total right hepatic lobectomy with concomitant nodal dissection for GBC (9). Given the lack of consensus, the surgical management of GBC remained controversial for many years. However, it was only until recent years that a more radical approach utilizing partial hepatic resection of the gallbladder bed with regional lymphadenectomy was advocated as standard approach for the treatment of GBC (10-12). In addition to surgical cholecystectomy with resection of gallbladder fossa (wedge hepatectomy of liver segments 4b and 5) and portal lymphadenectomy, advanced disease may necessitate major hepatectomy, resection of adjacent organs such as the pancreas, and biliary tree reconstruction. Currently, the National Comprehensive Cancer Network (NCCN recommends radical cholecystectomy with en bloc hepatic resection and regional lymph node dissection (LND) including porta hepatis, hepatoduodenal ligament, and retroduodenal nodes (13). Bile duct resection can be performed at the time of radical surgery if the tumor is resectable. For T1 tumors, simple cholecystectomy is considered curative and no further radical resection is warranted (10).
The number of LN removed has been shown to confer a substantial survival benefit in several gastrointestinal malignancies (14-18). For GBC, nodal clearance has been shown to decrease cancer-related mortality (19). In fact, nodal involvement is the strongest prognostic factor associated with long-term survival in patients undergoing radical resection for GBC. However, the extent of lymph node (LN) clearance has not been well established and remains a subject of debate. The objective of this study is to evaluate impact of more extensive LND in GBC. Using a national population-based database, the aim of this study is to determine the impact of extended LND on survival.
Methods
The Surveillance, Epidemiology, and End Results (SEER) database is a population-based database that collects cancer incidence and survival data, consisting of approximately 28% of the United States population. The SEER database started in 1973 and has been maintained by the National Cancer Institute. Available data include patient demographics, stage, tumor features, surgery, radiation therapy, LN examination and involvement, follow-up, and cause of death. Data from the SEER program released in 2012 was utilized in this study. The SEER database was queried to identify patients diagnosed with GBC from 1988 to 2009. Patients with gallbladder adenocarcinoma were identified based on site (C23.0). Stage was based on the 7th edition of the American Joint Commission on Cancer Staging Manual (20). The determination of stage was based on TNM, LN, and other extent of disease (EOD) data fields available in the SEER database. GBC diagnosed at autopsy was excluded from our analysis.
Summary statistics were performed to determine association between patient characteristics and LN evaluation. Age, sex, tumor size, LN evaluation, TNM stage, radiation therapy, and surgery were included in the analysis. Overall survival (OS) and cancer-specific survival (CSS) for stage I-IV were determined using Kaplan-Meier method and compared using log-rank test. Cox proportional hazard modeling was performed to determine prognostic factors. Minimal LND was defined having 1 to 3 LN evaluated and optimal LND was defined as having four or more LNs evaluated (≥4 LN). Patients with stage IV or metastatic disease were excluded from multivariate survival analysis. Statistical analysis was performed using SPSS version 19.0 (IBM Inc., Chicago, IL, USA). All tests were two-tailed. Statistical significance was set at P<0.05.
Results
A total of 12,990 patients diagnosed between 1988 and 2009 were identified, of whom 11,816 patients (91%) had adequate information in the SEER database to determine clinical stage. Cancer-directed surgery, defined as simple cholecystectomy or radical resection, was performed in 8,436 out of 11,816 patients (71.3%).
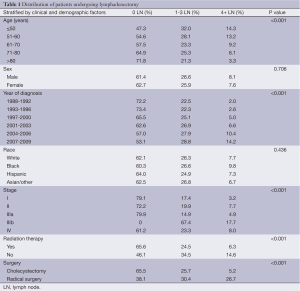
Patient and tumor characteristics in our patient population are detailed in Table 1. The mean age of diagnosis was 71 years (median 72 years). More extensive LND was only performed in 3.3% of patients greater than 80 years, 6.1% of patients between 71 and 80 years, 9.2% of patients between 61 and 70 years, 13.2% of patients between 51 and 60 years, and 14.3% of patients younger than 50 years. The majority of patients were females. The distribution of patients based on EOD was 20.2%, 18.5%, 22.2%, and 14.8%, and 24.5% for stage I, II, IIIa, IIIb, and IV, respectively. The mean and median tumor sizes were 3.2 and 2.5 cm, respectively.
Full table
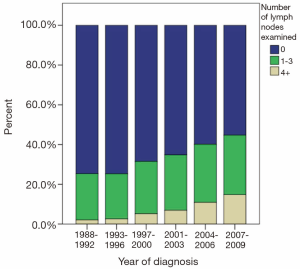
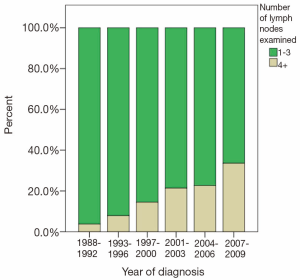
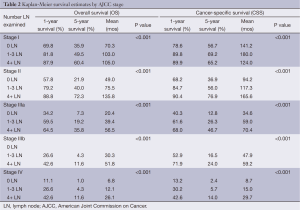
The majority of patients undergoing cancer-directed surgery did not undergo LND (62.3%). Greater LND was associated with young age, advanced T-stage, no radiation therapy, and radical surgery. Fewer than 10% of stage I-IIIa patients underwent extensive LND before 2004 (Figure 1). A greater proportion of patients diagnosed with stage IIIb disease underwent more extensive LND in recent years (Figure 2). The mean number of LND evaluated among stage I-IIIb patients was 2.7. No LNs were recovered in 79.4%, 77.5%, and 80% of patients with stage I, II, and IIIa, respectively. Minimal LND was performed in 17.4% of stage I, 19.9% of stage II, 14.9% of stage IIIa, and 67.4% of stage IIIb patients. More extensive LND was performed in 3.2% of stage I, 7.7% of stage II, 4.9% of stage IIIa, and 17.7% of stage IIIb patients. In all stages, greater LND was associated with longer 5-year cancer specific survival and OS (Table 2).
Full table
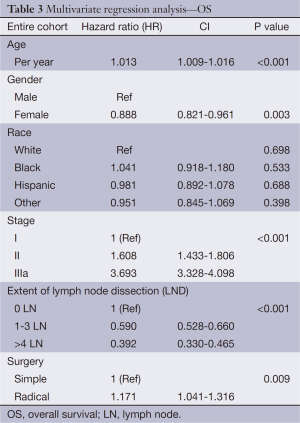
In order to determine the impact of LN clearance in patients with node-negative (stage I-IIIa) disease, multivariate analysis using Cox regression hazard modeling was performed. This revealed that early stage, removal of ≥4 LN, and radical resection were the most important independent predictors of survival (Table 3). However, subgroup analysis on stage I patients demonstrated that extent of LND did not significantly reduce risk of cancer-related mortality [hazard ratio (HR) 0.854; CI, 0.496-1.470, P=0.570].
Full table
Discussion
Early diagnosis and surgical resection provide the only chance for a cure for early stage GBC. Since the mid-1990s, there has also been more evidence supporting aggressive hepatic resection with lymphadenectomy for GBC (10,12,21). However, the extent of LND remains a subject of debate for GBC. In recent years, the number of recommended LND for GBC ranges from 1 to 6 LN (19,20,22-24). In the current study, we demonstrated that a greater number of LNs examined correlated with improved survival. Despite some survival benefit associated with greater LND, we found that LND remains underperformed in the United States. Only 7.7% patients had 4 or more LN examined, whereas 62.3% had no LND and 26.1% had minimal LND (1-3 nodes removed). Inadequate LN will likely increase the risk of locoregional recurrence as well as lead to inaccurate GBC staging and stage migration.
The importance of adequate LN assessment in accurate staging and survival is shown in other gastrointestinal malignancies. Current recommendations for colon cancer require sampling a minimum of 12 LN whereas other several studies suggest that more than 12 LNs (25-28). For pancreatic cancer, recent studies suggest that sampling at least 15 LN is required for accurate staging of pancreatic adenocarcinoma (29,30). As nodal involvement is a known poor prognostic factor for GBC, there is an increasing need to perform adequate lymphadenectomy in order to improve staging and identify node-positive patients who may benefit from adjuvant therapy after curative resection.
Currently, no consensus has been established regarding the appropriate extent of resection for GBC. Several series advocate for hepatic resection and regional LND over simple cholecystectomy to avoid residual disease and positive margins (11,31). More aggressive surgical interventions, including major hepatectomy and biliary tree reconstruction, have been advocated in some studies (10,32,33). While major hepatic resection is not mandatory in all GBC cases, it is still unclear if non-anatomic resection of segments IVb and V affects GBC survival. Theoretically, dissection of the gallbladder off the liver during simple cholecystectomy may violate the subserosal plane, thereby leaving behind microscopically positive disease (34). However, D’Angelica et al. suggested that the extent of resection did not necessarily improve survival outcomes; instead, survival is dictated by tumor biology and stage (11). Pawlik et al. suggested margin status was associated with survival, not extent of anatomical or non-anatomical hepatectomy (34). Furthermore, it is unclear whether or not radical resection is worthwhile for early T1 GBC. While some consider simple cholecystectomy as curative for early T1 disease, a recent study advocates for concomitant LND for early T1 disease (35). While the extent of LND was an independent predictor of survival in the current study, we noted similar survival rates in stage I patients who had either 1-3 or greater than 4 LN examined. Given the conflicting reports in the literature, further investigation is warranted to determine the value of radical cholecystectomy with lymphadenectomy for early GBC.
There are limitations to our study. The details on the extent of tumor resection (i.e., extent of hepatectomy, biliary tree reconstruction) are not available in the SEER database for GBC. Even though margin status is an important determinant of survival, margin status is not available in the current database. Data on recurrence and chemotherapy are also not available in the SEER database. Furthermore, although we assessed only node-negative patients in our multivariate survival analysis, stage migration remains a critical mechanism for the profound effect of the total number of LNs examined on survival. Nevertheless, our current analysis of the SEER database provides generalizable results on the role of lymphadenectomy for GBC.
In summary, more extensive lymphadenectomy (at least four or more nodes) correlates with improved survival in patients with GBC. Given the majority of patients with GBC are still undertreated in the US, greater efforts should be taken to retrieve adequate LN in patients with GBC. Given its prognostic importance, extent of lymphadenectomy will likely be an emerging quality metric for GBC in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Shih SP, Schulick RD, Cameron JL, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg 2007;245:893-901. [PubMed]
- Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001;51:349-64. [PubMed]
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006;118:1591-602. [PubMed]
- Kumar S, Kumar S, Kumar S. Infection as a risk factor for gallbladder cancer. J Surg Oncol 2006;93:633-9. [PubMed]
- Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst 1997;89:1132-8. [PubMed]
- Foster JM, Hoshi H, Gibbs JF, et al. Gallbladder cancer: Defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol 2007;14:833-40. [PubMed]
- Glenn F, Hays DM. The scope of radical surgery in the treatment of malignant tumors of the extrahepatic biliary tract. Surg Gynecol Obstet 1954;99:529-41. [PubMed]
- Pack GT, Miller TR, Brasfield RD. Total right hepatic lobectomy for cancer of the gallbladder; report of three cases. Ann Surg 1955;142:6-16. [PubMed]
- Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg 1996;224:639-46. [PubMed]
- D’Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol 2009;16:806-16. [PubMed]
- Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg 2000;232:557-69. [PubMed]
- Benson AB 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009;7:350-91. [PubMed]
- Bunt AM, Hermans J, Smit VT, et al. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol 1995;13:19-25. [PubMed]
- Caplin S, Cerottini JP, Bosman FT, et al. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer 1998;83:666-72. [PubMed]
- House MG, Fong Y, Arnaoutakis DJ, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg 2008;12:270-8. [PubMed]
- Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007;141:610-8. [PubMed]
- Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 2001;19:157-63. [PubMed]
- Jensen EH, Abraham A, Jarosek S, et al. Lymph node evaluation is associated with improved survival after surgery for early stage gallbladder cancer. Surgery 2009;146:706-11; discussion 711-3. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al, editors. American Joint Committee on Cancer: AJCC Cancer Staging Manual. 7th edn. New York: Springer, 2010.
- Dixon E, Vollmer CM Jr, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg 2005;241:385-94. [PubMed]
- Ito H, Ito K, D’Angelica M, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg 2011;254:320-5. [PubMed]
- Negi SS, Singh A, Chaudhary A. Lymph nodal involvement as prognostic factor in gallbladder cancer: location, count or ratio? J Gastrointest Surg 2011;15:1017-25. [PubMed]
- Coburn NG, Cleary SP, Tan JC, et al. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg 2008;207:371-82. [PubMed]
- Chen SL, Bilchik AJ. Resecting lymph nodes in colon cancer: more than a staging operation? Ann Surg Oncol 2007;14:2175-6. [PubMed]
- Chen SL, Steele SR, Eberhardt J, et al. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg 2011;253:82-7. [PubMed]
- Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003;21:2912-9. [PubMed]
- Wong JH, Severino R, Honnebier MB, et al. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol 1999;17:2896-900. [PubMed]
- Tomlinson JS, Jain S, Bentrem DJ, et al. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Arch Surg 2007;142:767-723; discussion 773-4.
- Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 2002;236:355-66; discussion 366-8. [PubMed]
- Yamaguchi K, Tsuneyoshi M. Subclinical gallbladder carcinoma. Am J Surg 1992;163:382-6. [PubMed]
- Fong Y. Aggressive therapy is warranted for gallbladder cancer. Cancer Invest 1998;16:64-5. [PubMed]
- Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive reresection is beneficial. Cancer 1998;83:423-7. [PubMed]
- Pawlik TM, Gleisner AL, Vigano L, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg 2007;11:1478-86; discussion 1486-7. [PubMed]
- Hari DM, Howard JH, Leung AM, et al. A 21-year analysis of stage I gallbladder carcinoma: is cholecystectomy alone adequate? HPB (Oxford) 2013;15:40-8. [PubMed]