Consensus and conflict in invasive micropapillary carcinoma: a case report and review of the literature
Introduction
As a distinct histologic type, invasive micropapillary carcinoma (IMPC) most commonly involves breast, urinary bladder, lung, ovary and colorectum. Other less common anatomic sites include ureter, renal pelvis, ampulla of Vater, pancreas, salivary gland, thyroid, stomach, gallbladder and bile duct (1-6). It is intriguing that, to the best of our knowledge, IMPC has never been reported in esophagus, liver and endometrium, except metastatic cases.
IMPC of the colorectum was first reported in 2005 (7). The incidence varies from 9.4% to 19.1% of colorectal carcinoma (8-13), much higher than the incidence of 3% to 6% for invasive breast carcinoma (14), 0.6% to 8.2% for invasive urothelial carcinoma (15) and 4% for carcinomas in the pancreatic/periampullary region (1). For reasons unknown, colorectal IMPC has a predilection for rectum (8,13), followed by right colon (10,13). We herein present a case of rectal IMPC arising from a tubulovillous adenoma and report for the first time HER2 status in rectal IMPC.
Case porsentation
A 60-year-old woman presented to an outside facility complaining intermittent painless bright red bleeding per rectum for 1.5 years with increased frequency in the past 8 months. Past medical history was significant for polymyalgia rheumatica on chronic prednisone for 3 years. She had no family history of cancer. The patient underwent diagnostic colonoscopy. A 3 cm rectal mass was identified and biopsied. However, further resection was limited by active bleeding from the mass. She then transferred her care to us.
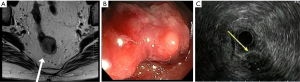
Reviewing her biopsy specimen confirmed the diagnosis of invasive moderately differentiated adenocarcinoma arising in tubulovillous adenoma. The tumor penetrated into muscularis mucosae. Focal lymphovascular invasion (LVI) was identified. Further workup showed serum carcinoembryonic antigen (CEA) was within normal range (2.9 ng/mL; reference 0.0-3.4 ng/mL). Magnetic resonance imaging (MRI) of pelvis reported a 3.0 cm × 2.5 cm × 1.9 cm mass located at the mid rectal left lateral wall (Figure 1A). It was isodense with muscle on the T2 images, and enhanced on post-contrast study. The mass involved the mucosa and submucosa with adjacent thinning of the muscular layer but no evidence of penetrating through the serosa. No pelvic lymphadenopathy was identified. The MRI diagnosis was rectal mass, stage T2N0M0. Flexible sigmoidoscopy and rectal ultrasonography revealed an exophytic mass (Figure 1B,C) located 15 cm from anal verge. It was removed by two cold snare resections.
The two pieces of specimen measured 2.2 cm × 1.5 cm × 1.0 cm and 1.5 cm × 1.0 cm × 1.0 cm, respectively. The cut surface was gray white. Hematoxylin-Eosin (H.E.) staining showed that the majority of the tumor was composed of irregularly shaped glands showing infiltrative growth pattern and intermediate to high grade nuclear features, consistent with moderately-differentiated adenocarcinoma (Figure 2A). Approximately 20% of the tumor consisted of small clusters of malignant cells floating in clear spaces (Figure 2B). Lumen formation within the clusters was occasionally seen. The tumor arose in a background of tubulovillous adenoma with high grade dysplasia, and extended into underlying muscularis mucosae. Surgical margins were involved.

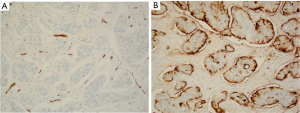
To differentiate micropapillary component (MC) from LVI, a panel of immunostaining was performed with adequate positive and negative controls. CD34 highlighted vasculature, with no evidence of LVI seen. The clear spaces surrounding tumor cell nests were CD34 negative (Figure 3A). CD10 decorated the outer surfaces of tumor nests, consistent with reversed polarity (Figure 3B). Luminal surfaces within the cluster were also stained. Epithelial membrane antigen (EMA) showed cytoplasmic and intercellular membranous positivity in the tumor nests, with minimal staining on the stromal facing surface. The final diagnosis was invasive moderately differentiated adenocarcinoma with MC. Immunostaining for HER2 showed weak incomplete membrane staining in less than 5% of the cells. Though there is no appropriate HER2 scoring system for colorectal cancer, weak incomplete membrane staining is interpreted as negative if the scoring system for breast cancer is applied.
Given the positive surgical margins and the T2 stage of the tumor, the patient was offered total mesorectal excision without neoadjuvant therapy. Macroscopically, there was a 2.0 cm × 1.5 cm area with central ulceration on the mucosal surface. Gross residual tumor was absent. The area was totally submitted for microscopic examination. H.E. stained sections showed ulceration and scarring only. No microscopic residual carcinoma was identified. Twenty-four lymph nodes examined were free of metastatic carcinoma. The patient had no evidence of disease 2 months post-surgery.
Discussion
Growth pattern of colorectal IMPC is highly variable and nonspecific. Verdú et al. reported that 70% of cases were ulcerated or stenosing, whereas 30% were polypoid or exophytic. Microscopically, 96.7% had infiltrative borders and only 3.3% showed expansive borders (12).
Histologically, IMPC is characterized by small clusters of cohesive neoplastic cells floating in lacunar spaces. It is called micropapillary other than papillary due to lack of fibrovascular cores. Tumor cells have moderate amount of eosinophilic cytoplasm, prominent cytoplasmic membrane and intermediate to high grade nuclei. The lacunar spaces are lined by delicate strands of fibrocollagenous stroma without endothelial lining. It is assumed that these spaces are retraction artifact. However, IMPC can occasionally be seen on frozen sections (16), suggesting these spaces may be real but exaggerated by routine histologic processing. The stroma typically has minimal to no desmoplastic response, except some cases arising in stomach and colon (4,17). IMPC is not associated with increased lymphocytic infiltrate (8,12). However, IMPC arising in ampullo-pancreatobiliary region shows peculiar neutrophil infiltrate, both intraepithelial and stromal, of unknown clinical significance (1).
Pure IMPC is extremely rare. IPMC often coexists with other histologic types, with a percentage ranging from 5% to 95% but usually less than 30% (13). It is more often seen in the deep advancing edge of the tumor, but can be seen in the center. The transition from IMPC to conventional adenocarcinoma may be abrupt or gradual.
The most distinguishing feature of IMPC is reversed polarity, also known as “inside-out” growth pattern. The basal stroma-facing surface displays secretory properties, mimicking the apical surface in conventional adenocarcinoma. Ultrastructurally, the basal surface is covered with numerous microvilli, and able to secrete into surrounding space as if it is facing a true lumen (18). The simplest way to demonstrate reversed polarity is immunohistochemistry, which has been used to verify the diagnosis of IMPC in many studies. MUC1 and EMA, the overall gold-standard markers for this purpose, are expressed on basal surfaces of tumor cell nests in IMPC, as opposed to apical surfaces of benign glands and malignant glands of conventional adenocarcinoma (19).
It is noteworthy that basal MUC1 staining, though very sensitive, is not specific for IMPC. Strong basal MUC1 staining can be seen in 63% of cases of invasive urothelial carcinoma with retraction artifact (20). Moreover, the staining pattern of MUC1/EMA is not very specific either, with cytoplasmic and non-basal membranous staining frequently seen (20-22). KL-6, an antibody against different epitope of MUC1, has been reported to give specific linear positivity without cytoplasmic staining in breast, gastric and urothelial carcinoma, but has not been widely tested and does not work for colorectal IMPC (21,23). On the other hand, negative basal MUC1/EMA staining does not preclude the diagnosis of IMPC in cases showing characteristic morphology. Colorectal IMPC, in particular, often shows negativity to focal positivity of MUC1/EMA (21,22,24). Other markers reported include CD10, villin, MUC3 and CEA in gastrointestinal tract (21,22) and CD15, CEA, KL-6, Her2Neu and CA125 in urinary tract (20,21).
In terms of pathogenesis, reversed polarity is believed to be responsible for the aggressive behavior of IMPC. MUC1 expression on apical surface contributes to lumen formation in normal glands, whereas MUC1 expression on basal surface disrupts cell adhesion to the stroma and results in characteristic lacunar spaces in IMPC. Moreover, secretory capability of the basal surface allows secretion of metalloproteinase into lacunar spaces that facilitates dissection of stroma and early LVI (19). It is intriguing that micropapillary serous carcinoma of the ovary displays similar MUC1 staining pattern but has indolent clinical course. Another proposed mucin family candidate is MUC2, which can form a physical barrier against tumor spread. Lack of MUC2 expression in IMPC therefore permits easier tumor dissemination (4,19). However, upregulation of MUC2 has not yet been documented in micropapillary serous carcinoma of the ovary. It remains unknown why MC in the ovary is not associated with aggressive clinical course.
Other observed abnormalities at the molecular level include aberrant expression of HER-2/neu and p53 (2,12), upregulation of insulin-like growth factor II mRNA-binding protein-3 (24), cyclin D1 and MYC (8q24) (25), and down-regulation and/or aberrant location of E-cadherin and β-catenin (1,8,24,26).
Molecular genetic study of IMPC is difficult partially because most of the cases have mixed histology. Colorectal IMPC, compared to conventional adenocarcinoma, has been reported to carry a higher frequency of TP53 mutation and/or accumulation as well as a lower incidence of microsatellite instability and RER phenotype, suggesting a classical chromosomal instability pathway in the pathogenesis of IMPC (12,13). In fact, genetic study of breast IMPC showed a very complex alteration, particularly high-level amplification of multiple regions on 8q (25,27).
A literature search retrieved one case of colonic IMPC arising from tubulovillous adenoma (28). Our case represents the first reported rectal IMPC arising from tubulovillous adenoma with high grade dysplasia. Whether the presence of adenoma increases the risk of IMPC is unknown. In the case series reported by Verdú et al., adenoma was found in 30% of colorectal IMPC cases, similar to that of conventional adenocarcinoma (12). Though the authors did not specify whether the adenomas were associated with IMPC or separate lesions, IMPC did not appear to have increased association with adenoma compared to conventional colorectal adenocarcinoma (12).
Given the similar morphology of IMPC arising from different organs, high propensity of LVI in IMPC as well as the fact that metastatic tumor often consists entirely of MC, determining the primary site of IMPC takes extra caution (29). When in doubt, an IHC panel of four markers including uroplakin, TTF-1, ER and WT-1 or PAX8 is suggested to cover common primary sites of IMPC (30).
Another diagnostic challenge is to differentiate nonclassic IMPC from extensive retraction artifact. IMPC has been reported to account for 0.07-13.4% of gastric cancer cases (4,31). The near 200-fold difference probably reflects the misinterpretation of gray-zone cases in some studies. Indeed, interobserver reproducibility is only moderate even among expert pathologists (15). Before the diagnostic criteria are refined to settle the disagreements, a new question emerges: is it necessary to differentiate IMPC from extensive retraction artifact? For some cancers such as basal cell carcinoma, retraction artifact is just one of the histologic features with no known clinical significance. However, extensive stromal retraction is not always an artifact that can be ignored. In breast carcinoma, extensive retraction artifact is also strongly associated with LVI, nodal metastasis and poor outcome, though to a slightly lesser extent than IMPC does (16). Basal MUC1 staining, an evidence of reversed polarity that is assumed to explain aggressive behavior of IMPC, is also seen in urothelial carcinoma with retraction artifact (20). If both stromal retraction and IMPC are reflections of altered tumor-stromal interaction which facilitates LVI and tumor spread, there is a possibility that extensive stromal retraction alone could be a simplified but more accurate prognostic feature than MC (15).
On the other hand, Acs et al. reported that 7.0% of invasive ductal carcinomas of the breast demonstrated tumor cell nests focally and slightly detached from the stroma. Immunostaining for EMA confirmed partial reversed polarity, in other words, linear reactivity on part of the periphery of tumor cell nests (32). Despite the superficial resemblance to IMPC, these features were also strongly associated with LVI and nodal metastasis, to the same extent as that of IMPC. The study on these “underdeveloped IMPC’’ cases suggests that reversed polarity, no matter complete or partial, may be the key to the increased risk of lymphatic spread.
An interesting study recently published by Barresi et al. showed that, in colorectal cancer, poorly-differentiated clusters (PDC) had not only significant morphologic overlap with MC, reversed polarity as evidenced by MUC1 immunostaining but also high propensity of LVI, perineural invasion and nodal metastases (33). The authors proposed that the term micropapillary be replaced by PDC because PDC reflects the underlying biological phenomenon and, more importantly, the new grading system based on PDC has better reproducibility and higher predictive value on clinical outcome. The only morphologic difference mentioned by the authors was, clear spaces around PDCs were less prominent than those in IMPC (33). The clinical significance of space prominence is uncertain at this time. It is noteworthy that, by definition, PDCs lack gland formation. In contrast, lumina within cell nests are not uncommon in IMPC. Lumen formation is a sign of differentiation associated with better prognosis. The presence or absence of lumina may be more critical difference that explains the better reproducibility of PDC.
Regarding prognosis, colorectal IMPC has 1, 3 and 5-year survival rates of 80%, 50%, and 37%, respectively; and 1, 3 and 5-year disease-free survival rates of 69%, 38%, and 33%, respectively (9). The prognostic significance of MC is particularly meaningful for early stage disease, as one would expect that probably nothing else matters to advanced-stage cancer. Xu et al. and Eom et al. reported separately that, compared to conventional adenocarcinoma, IMPC was associated with worse prognosis in TNM stages I to II colorectal and gastric cancers, but no difference was seen in TNM stages III to IV diseases (8,26). However, Lee et al. reported that the overall survival difference was only seen in stage III colorectal carcinoma (9). IMPC-associated poor prognosis has been largely attributed to increased risk of LVI, depth of invasion and hence higher stages. Whether or not the histology by itself has any independent prognostic value remains a topic of debate. Interestingly, Xu et al. reported that stage I to II colorectal IMPC had similar survival rates as stage III to IV cases (8). In other words, MC may trump stage in terms of prognosis. Of note, there are some exceptions to IMPC-associated poor prognosis. As discussed earlier, IMPC of the ovary is a low grade lesion for some reasons. The prognosis of gastric IMPCs was same as that of conventional tubular or papillary adenocarcinoma in one study (4). The prognostic significance of micropapillary features is unclear in prostatic ductal adenocarcinoma.
Whether the percentage of MC affects prognosis in proportion is controversial. Though the presence of MC mattered, the proportion of MC did not make any further difference in terms of nodal metastasis and survival according to the majority of the studies (4,9,11-13,16,34). However, a couple of large-scale studies claimed that rate of lymph node metastasis positively did correlate with the percentage of MC (24,35). There is a possibility that the sample size needs to be large enough to reach statistical significance. Anyway, irrespective of the proportions of MC in the primary tumors, the metastases consist of predominant or exclusive MC. Therapeutically, the presence of MC in urothelial carcinoma prompts radical cystectomy, no matter how small the percentage is.
The mainstay of the treatment is surgery. IMPC is resistant to chemotherapy and radiation (36), possibly due to stem cell-like features of tumor cells (9,26). Whether HER2-targeted therapy can to some extent alter the dismal clinical course awaits further study. Over 95% of breast IMPCs demonstrated HER2 overexpression. Next generation sequencing identified activating HER2 mutation in 40% of micropapillary urothelial carcinoma cases (37). Moreover, HER2 gene amplification was associated with a nearly threefold increased risk of cancer death in micropapillary urothelial carcinoma, and helped identify patients with poor outcome (38). In gastric IMPC, HER2 gene amplification and protein overexpression have been documented (23,39). So far, HER2 status in colorectal IMPC has never been reported. To the best of our knowledge, our case report represents the first study of HER2 expression in rectal IMPC and shows no evidence of HER2 overexpression, although study on more cases is warranted to draw a conclusion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Khayyata S, Basturk O, Adsay NV. Invasive micropapillary carcinomas of the ampullo-pancreatobiliary region and their association with tumor-infiltrating neutrophils. Mod Pathol 2005;18:1504-11. [PubMed]
- Nagao T, Gaffey TA, Visscher DW, et al. Invasive micropapillary salivary duct carcinoma: a distinct histologic variant with biologic significance. Am J Surg Pathol 2004;28:319-26. [PubMed]
- Chernock RD, El-Mofty SK, Becker N, et al. Napsin A expression in anaplastic, poorly differentiated, and micropapillary pattern thyroid carcinomas. Am J Surg Pathol 2013;37:1215-22. [PubMed]
- Roh JH, Srivastava A, Lauwers GY, et al. Micropapillary carcinoma of stomach: a clinicopathologic and immunohistochemical study of 11 cases. Am J Surg Pathol 2010;34:1139-46. [PubMed]
- Hara S, Kijima H, Okada K, et al. Invasive micropapillary variant of the gallbladder adenocarcinoma and its aggressive potential for lymph node metastasis. Biomed Res 2010;31:89-95. [PubMed]
- Yoshizawa T, Toyoki Y, Hirai H, et al. Invasive micropapillary carcinoma of the extrahepatic bile duct and its malignant potential. Oncol Rep 2014;32:1355-61. [PubMed]
- Sakamoto K, Watanabe M, De La Cruz C, et al. Primary invasive micropapillary carcinoma of the colon. Histopathology 2005;47:479-84. [PubMed]
- Xu F, Xu J, Lou Z, et al. Micropapillary component in colorectal carcinoma is associated with lymph node metastasis in T1 and T2 Stages and decreased survival time in TNM stages I and II. Am J Surg Pathol 2009;33:1287-92. [PubMed]
- Lee HJ, Eom DW, Kang GH, et al. Colorectal micropapillary carcinomas are associated with poor prognosis and enriched in markers of stem cells. Mod Pathol 2013;26:1123-31. [PubMed]
- Lino-Silva LS, Salcedo-Hernández RA, Caro-Sánchez CH. Colonic micropapillary carcinoma, a recently recognized subtype associated with histological adverse factors: clinicopathological analysis of 15 cases. Colorectal Dis 2012;14:e567-72. [PubMed]
- Haupt B, Ro JY, Schwartz MR, et al. Colorectal adenocarcinoma with micropapillary pattern and its association with lymph node metastasis. Mod Pathol 2007;20:729-33. [PubMed]
- Verdú M, Román R, Calvo M, et al. Clinicopathological and molecular characterization of colorectal micropapillary carcinoma. Mod Pathol 2011;24:729-38. [PubMed]
- Kim MJ, Hong SM, Jang SJ, et al. Invasive colorectal micropapillary carcinoma: an aggressive variant of adenocarcinoma. Hum Pathol 2006;37:809-15. [PubMed]
- Tavassoli FA, Devilee P, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press, 2003.
- Sangoi AR, Beck AH, Amin MB, et al. Interobserver reproducibility in the diagnosis of invasive micropapillary carcinoma of the urinary tract among urologic pathologists. Am J Surg Pathol 2010;34:1367-76. [PubMed]
- Acs G, Paragh G, Chuang ST, et al. The presence of micropapillary features and retraction artifact in core needle biopsy material predicts lymph node metastasis in breast carcinoma. Am J Surg Pathol 2009;33:202-10. [PubMed]
- Kuroda N, Oonishi K, Ohara M, et al. Invasive micropapillary carcinoma of the colon: an immunohistochemical study. Med Mol Morphol 2007;40:226-30. [PubMed]
- Luna-Moré S, Gonzalez B, Acedo C, et al. Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract 1994;190:668-74. [PubMed]
- Nassar H, Pansare V, Zhang H, et al. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol 2004;17:1045-50. [PubMed]
- Sangoi AR, Higgins JP, Rouse RV, et al. Immunohistochemical comparison of MUC1, CA125, and Her2Neu in invasive micropapillary carcinoma of the urinary tract and typical invasive urothelial carcinoma with retraction artifact. Mod Pathol 2009;22:660-7. [PubMed]
- Ohtsuki Y, Kuroda N, Umeoka T, et al. KL-6 is another useful marker in assessing a micropapillary pattern in carcinomas of the breast and urinary bladder, but not the colon. Med Mol Morphol 2009;42:123-7. [PubMed]
- Cserni G. Reversed polarity of the glandular epithelial cells in micropapillary carcinoma of the large intestine and the EMA/MUC1 immunostain. Pathology 2014;46:527-32. [PubMed]
- Ohtsuki Y, Kuroda N, Yunoki S, et al. Immunohistochemical analysis of invasive micropapillary carcinoma pattern in four cases of gastric cancer. Med Mol Morphol 2013;46:114-21. [PubMed]
- Liu F, Sun AJ, Sun LP, et al. Gastrointestinal adenocarcinomas with a micropapillary pattern: a clinicopathologic and immunohistochemical study. Zhonghua Bing Li Xue Za Zhi 2011;40:304-9. [PubMed]
- Marchiò C, Iravani M, Natrajan R, et al. Genomic and immunophenotypical characterization of pure micropapillary carcinomas of the breast. J Pathol 2008;215:398-410. [PubMed]
- Eom DW, Kang GH, Han SH, et al. Gastric micropapillary carcinoma: A distinct subtype with a significantly worse prognosis in TNM stages I and II. Am J Surg Pathol 2011;35:84-91. [PubMed]
- Marchiò C, Iravani M, Natrajan R, et al. Mixed micropapillary-ductal carcinomas of the breast: a genomic and immunohistochemical analysis of morphologically distinct components. J Pathol 2009;218:301-15. [PubMed]
- Kondo T. Colon invasive micropapillary carcinoma arising in tubulovillous adenoma. Pol J Pathol 2008;59:183-5. [PubMed]
- Ramalingam P, Middleton LP, Tamboli P, et al. Invasive micropapillary carcinoma of the breast metastatic to the urinary bladder and endometrium: diagnostic pitfalls and review of the literature of tumors with micropapillary features. Ann Diagn Pathol 2003;7:112-9. [PubMed]
- Lotan TL, Ye H, Melamed J, et al. Immunohistochemical panel to identify the primary site of invasive micropapillary carcinoma. Am J Surg Pathol 2009;33:1037-41. [PubMed]
- Lee JH, Kim JH, Choi JW, et al. The presence of a micropapillary component predicts aggressive behaviour in early and advanced gastric adenocarcinomas. Pathology 2010;42:560-3. [PubMed]
- Acs G, Esposito NN, Rakosy Z, et al. Invasive ductal carcinomas of the breast showing partial reversed cell polarity are associated with lymphatic tumor spread and may represent part of a spectrum of invasive micropapillary carcinoma. Am J Surg Pathol 2010;34:1637-46. [PubMed]
- Barresi V, Branca G, Vitarelli E, et al. Micropapillary pattern and poorly differentiated clusters represent the same biological phenomenon in colorectal cancer: a proposal for a change in terminology. Am J Clin Pathol 2014;142:375-83. [PubMed]
- Nassar H, Wallis T, Andea A, et al. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol 2001;14:836-41. [PubMed]
- Tang T, Zhang XJ, Zhao LZ, et al. Relationship between colorectal adenocarcinoma with invasive micropapillary carcinoma component and lymph node metastasis. Zhonghua Bing Li Xue Za Zhi 2013;42:525-9. [PubMed]
- Alvarado-Cabrero I, Alderete-Vázquez G, Quintal-Ramírez M, et al. Incidence of pathologic complete response in women treated with preoperative chemotherapy for locally advanced breast cancer: correlation of histology, hormone receptor status, Her2/Neu, and gross pathologic findings. Ann Diagn Pathol 2009;13:151-7. [PubMed]
- Ross JS, Wang K, Gay LM, et al. A high frequency of activating extracellular domain ERBB2 (HER2) mutation in micropapillary urothelial carcinoma. Clin Cancer Res 2014;20:68-75. [PubMed]
- Schneider SA, Sukov WR, Frank I, et al. Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Mod Pathol 2014;27:758-64. [PubMed]
- Tajima S, Kodama H, Kamiya T, et al. Gastric carcinoma with an invasive micropapillary carcinoma component showing HER2 gene amplification and CD10 expression: a case report and review of the literature. Pathol Int 2014;64:402-8. [PubMed]


