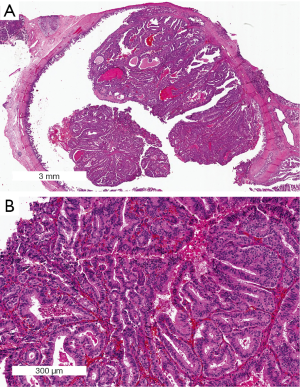
Pancreatic adenocarcinoma pathology: changing “landscape”
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease with an extremely poor prognosis. The overall 5-year survival rate is 6%. The median survival varies from almost 2 years for patients with local and resectable disease, to only a few months for patients with advanced metastatic disease. Unfortunately, the vast majority of patients present at an advanced inoperable stage, whereas only about 20% of patients have localized disease that is amenable for surgery (1). To improve prognosis for patients with PDAC, it is essential to diagnose and treat the disease in the earliest stages, ideally even before a full blown invasive PDAC is established, by treating precursor lesions (2).
A growing body of evidence has helped establish that invasive PDAC develops from well-defined noninvasive precursor lesions (3). The most common precursor to invasive PDAC, pancreatic intraepithelial neoplasia (PanIN), is microscopic (3). In addition to this microscopic lesion, there are two macroscopically discernible cystic precursor lesions in the pancreas (4). These cystic precursor lesions are intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) (4).
In addition to morphologic characterization of pancreatic carcinogenesis, our understanding of the genetic alterations that drive carcinogenesis has increased dramatically over the last decades. In particular, recent advancements in sequencing technologies have immensely deepened our understanding of the genetics of PDAC (5-9). Whereas earlier studies have focused on the major driver genes involved in invasive PDAC, more recent studies using next-generation sequencing (NGS) have produced a more complete understanding of the genetics of PDAC, its variants, and its precursor lesions. Mathematical modeling of genetic data suggests that the genetic evolution of PDAC takes almost 12 years from the earliest genetic alteration in a precursor lesion to the development of a full-blown invasive cancer (10). Thus there is an almost 12-year window of opportunity to prevent PDAC from even developing if we can identify and treat noninvasive precursor lesions. In addition, the genes targeted in pancreatic neoplasms may serve as future biomarkers in the genetic diagnosis of PDAC and its precursors (7,8). This review article discusses the pathology and the current knowledge of genetics of PDAC and its precursor lesions PanIN, IPMN and MCN.
Genetics of invasive PDAC and its precursor lesions
Genetics of invasive PDAC
Invasive PDAC is one of the best understood tumors at the genetic level (5-7,10,11). Invasive PDACs are genetically very complex, with wide-spread chromosome abnormalities, numerous losses and gains of large segments of DNA, and on average more than 60 exomic alterations in each cancer (12,13). The genes most commonly targeted in PDAC are KRAS, CDKN2A, TP53 and SMAD4. In addition, several less commonly mutated genes, including MLL3, SMAD3, FBXW7 and ARID1A have been identified. Germline mutations in BRCA2 and CDKN2A, and less frequently in BRCA1, PALB2 and ATM have been identified in a small subset of patients with familial PDAC (14-16). In addition, patients with Lynch syndrome (caused by germline mutation in one of the mismatch repair genes MLH1, MSH2, MSH6 or PMS2) and Peutz-Jeghers syndrome (PJS) (caused by germline mutation of the STK11 gene) are at increased risk of PDAC (17,18).
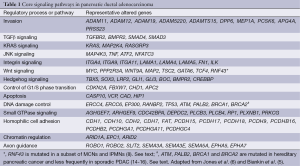
Importantly, despite the relatively large number of genes targeted in PDAC, genetic alterations in PDAC have been shown to involve several core cellular signaling pathways and processes (Table 1).These include chromatin modification (EPC1 and ARID2), DNA damage repair (TP53, ATM, PALB2 and BRCA2) and other mechanisms (ZIM2, MAP2K4, NALCN, SLC16A4 and MAGEA6) (6). In addition, a recent study has also suggested that genes described traditionally as embryonic regulators of axon guidance, particularly signaling trough slit ligands and roundabout receptors (SLIT/ROBO), may also be targeted in pancreatic cancer (5). Most PDACs harbor a mutation in a gene in each core pathway, but the specific gene mutated in a given pathway can differ among different PDACs. Therapeutic targeting of one or more of these pathways may thus be more effective than targeting of a specific genetic alteration.
Full table
With the advances in sequencing technologies, the genetic alterations in PDAC can now be studied at unprecedented levels, providing insights into the disease in ways that simply were not possible a decade ago. For example, comparisons of the genetic alterations in metastases to the primary tumors from which they arose provided insight into the length of time it takes for metastases to develop. Yachida et al. found that the genetic alterations in metastatic PDACs are surprisingly similar to those in matched primary tumors (7). By investigating whether mutations identified in the index metastasis were present or absent in multiple additional samples from the primary tumor they identified two categories of mutations. First, mutations present in all samples from a given patient were considered “founder mutations”, which were likely established in the noninvasive precursor lesion that gave rise to the invasive PDAC. Founder mutations included mutations in the major genes known to be involved in pancreatic carcinogenesis (i.e., KRAS, CDKN2A, TP53, and SMAD4). Mutations that were only present in a subset of the samples from each patient were considered “progressor mutations”. Progressor mutations occurred later than founder mutations and represent subclonal evolution beyond the parental clone. Of interest, Yachida et al. found that clonal populations that give rise to distant metastases were represented within the primary carcinoma, but these clones were genetically evolved from the original parental, nonmetastatic clone. Thus, genetic heterogeneity of metastases reflects the heterogeneity within the primary carcinoma. Extending this observation further using quantitative analyses of the timing of the genetic evolution of PDAC, Yachida and colleagues calculated that almost 12 years pass between the initiating mutation and the birth of the nonmetastatic invasive PDAC. Five more years are required for the acquisition of metastatic ability and the average patient dies 2 years thereafter (7). Compared to the traditional view on PDAC as a very rapidly progressing disease that is almost instantaneously metastatic, these studies revealed that genetic evolution and growth of PDAC resembles that of other tumor types and that there is a wide window of opportunity for early detection and treatment (10).
Pancreatic intraepithelial neoplasia (PanIN)
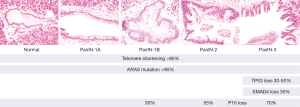
The vast majority of PDACs are believed to arise from PanIN (3,19). PanINs are small microscopic lesions that are <5 mm. They are composed of a flat or papillary neoplastic epithelium. Three grades of dysplasia can distinguished in PanIN lesions (Figure 1). PanIN-1A and PanIN-1B have low-grade dysplasia. They are characterized by tall columnar cells with basally located small round-to-oval nuclei and abundant supranuclear mucin. PanIN-1A has flat epithelium, whereas PanIn-1B is characterized by papillary or micropapillary architecture. PanIN-2 is considered intermediate-grade dysplasia and shows mostly papillary epithelium with mild to moderate cytological atypia. PanIN-3 is considered high-grade dysplasia (carcinoma in situ) and characterized by usually papillary or micropapillary proliferations of cells with significant cytological atypia (19). Of note, PanINs are often surrounded by lobular parenchymal atrophy which, when multifocal, can be detected by endoscopic ultrasound and may serve as a biomarker in patients at high-risk for PDAC (20).
PanIN lesions are common in the pancreas. For example, Konstantinidis and colleagues found PanINs in 153 (26%) of 584 pancreata surgically resected for a reason other than PDAC. Most of these lesions were PanIN-1 (50% of pancreata with PanIN) and PanIN-2 (41% of pancreata with PanIN), whereas PanIN-3 was only present in 13 cases (8% of pancreata with PanIN) (21). By contrast PanIN-3 has been reported to be present in 30-50% of pancreata with an invasive PDAC (19). Moreover, the number of PanINs, in particular those with high-grade dysplasia, is higher in patients with a strong family history of PDAC compared to patients with a PDAC but no family history of the disease (22).
Genetic studies support the hypothesis that PanINs can be a precursor to invasive pancreatic cancer, and have shown that the increasing morphologic grades of dysplasia in PanIN are accompanied by the accumulation of genetic alterations (Figure 1) (3). Telomere shortening and activating mutations in the KRAS oncogene are the most common alterations in low-grade PanIN lesions (23-25). Studies in genetically modified mouse models have shown that KRAS mutations can initiate PanIN development (26), and deep sequencing using NGS techniques have shown that KRAS mutations are present in >90% of all PanIN lesions, even those with low-grade dysplasia. These deep sequencing studies suggest a gradual expansion of the KRAS-mutant clone during PanIN progression (24). It appears that KRAS mutation alone provides only a modest selective advantage over neighboring cells and that additional genetic or epigenetic events are needed for neoplastic progression (24).
A subset of PanINs (10%) harbors a GNAS mutation, a recently discovered oncogene mutated in about 60% of IPMNs (9,24). Interestingly, in some PanINs a GNAS mutation is the only mutation and in other PanINs the GNAS mutation seems to have occurred earlier than the KRAS mutation. Some of these PanIN lesions with a GNAS mutation may progress to IPMNs, as Matthaei and colleagues found that 33% of lesions with a size between PanINs and IPMNs (the so called incipient IPMNs) harbor GNAS mutations (27). Together these data suggest that GNAS mutations in PanIN may drive the lesion towards the IPMN pathway, although specificity of GNAS mutations for the IPMN pathway needs further confirmation.
The other genes targeted in invasive PDAC, including CDKN2A/P16, TP53 and SMAD4, are also altered in PanIN lesions, supporting the hypothesis that PanINs are a precursor to invasive PDAC (3,28-30). These genetic alterations appear to occur after telomere shortening and KRAS gene mutations, as they are usually not found in low-grade PanINs, but instead are found in higher-grade PanIN lesions.
Some of the genetic changes in PanINs appear to be associated with progression (24). For example, loss of P16 protein expression, a marker for genetic inactivation of CDKN2A/P16, correlates with increasing PanIN grade (30% of PanIN-1A/B, 55% of PanIN-2, and 70% of PanIN-3 lost P16 expression) (28,31).This finding suggests that loss of P16 may be more important for progression of PanIN than for initiation (3,24). Late genetic events that almost exclusively occur in PanIN-3 are inactivation of TP53 and SMAD4, which are found in 30-50% of PanIN-3 lesions (29,30).
In addition to genetic changes, epigenetic alterations also play a role in PanIN progression. Hypermethylation of the promoters of tumor suppressor genes can be seen in low-grade PanIN lesions and they increase with grade of dysplasia (32). Promoter hypermethylation of CDKN2A/P16 is responsible for a third of P16 silencing, whereas homozygous deletions and intragenic mutation coupled with loss of heterozygosity (LOH) account for the remaining two-thirds (31). Many microRNAs are aberrantly expressed in PanINs and some of these are likely to be important in pancreatic carcinogenesis. Expression of some microRNAs, such as miR-196b, appears specific for high-grade lesions (PanIN-3 and PDAC) (24).
The genetic alterations, if any, that are crucial for transition from high-grade PanIN (in situ carcinoma) to an invasive carcinoma are still largely unknown. Direct comparative sequencing of a precursor lesion and the associated invasive carcinoma can greatly increase our knowledge of the genetic changes that drive this transition. However, it is almost impossible to identity the exact PanIN that gave rise to the PDAC since much of the pancreas is usually overgrown by the PDAC once the tumor is resected. Also distinction between PanIN-3 adjacent to PDAC and the process of “cancerization of a pancreatic duct” by a PDAC can be difficult (19). Despite these difficulties, Murphy and colleagues tried to address the mechanisms that control progression to invasion by exome sequencing of 10 PDACs and 15 adjacent PanIN-2 and PanIN-3 lesions (33). PanINs and invasive carcinomas appeared to harbor similar numbers of mutations. There was a trend towards fewer mutations in PanIN-2 (average of 30 mutations) compared to the invasive carcinomas (average 50 mutations), but, surprisingly, PanIN-3 showed on average more mutations (63 mutations). In total, 66% of mutations were common to the invasive carcinoma and the adjacent PanIN, 10% of mutations were only present in the invasive carcinoma, and 25% of the mutations were only present in the PanIN lesions. When individual PanIN lesions were analyzed, genetic overlap between PanIN and adjacent invasive carcinoma ranged from 34% to 96%, but >50% commonality of mutations was present in 10 of the 15 PanIN lesions (33). The very high commonality between PanIN and invasive carcinoma in a few cases may represent very recent genetic divergence, but also raises the concern that a lesion is actually ductal spread of the adjacent invasive cancer instead of a true PanIN-3 lesion.
A number of clinical studies of PanIN lesions have been performed in parallel to the previously mentioned genetic studies, and the clinical significance of PanIN lesions in different settings is now being understood. PanIN at a resection margin does not affect survival in patients who have a resection for invasive PDAC. This is likely because the patients with invasive cancer and a PanIN at a margin are likely to die from their invasive PDAC long before the residual PanIN has time to progress to an invasive cancer (34). Although the data are not so strong, Konstantinidis and colleagues investigated the significance of incidentally discovered PanIN in pancreatic resections for reasons other than PDAC (21). They found that presence of PanIN-1 or 2 in the resection margin or PanIN of any grade anywhere in the pancreas did not result in an appreciable cancer risk in the pancreatic remnant after resection (21). Follow-up of patients in this study was relatively short [median 3 years (range, 0.5-11 years)] compared to the time needed for PDAC development (11,21).
Intraductal papillary mucinous neoplasm (IPMN)
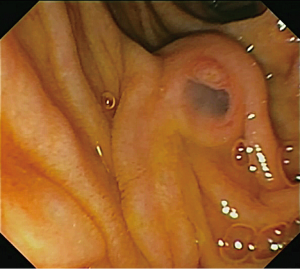
IPMNs are epithelial mucin-producing tumors that arise within the larger pancreatic ducts. At endoscopy a so-called “fish-eye” ampulla of Vater, i.e., a bulging ampulla with extruding mucin, can be seen and is almost diagnostic for IPMN (Figure 2). IPMNs are by definition >5 mm in diameter, and they typically are characterized by papillary proliferations that dilate the existing duct infrastructure. IPMNs are usually found in the head of the pancreas, but they can involve any portion of the pancreas and some involve the entire length of the gland (35,36). IPMNs are very common, and studies of asymptomatic individuals who undergo a CT scan have revealed that close to 3% of asymptomatic individuals have pancreatic cysts, approximately 25% of which is consistent with an IPMN (35,37,38). The prevalence of IPMN is equal in men and women; the majority of patients are diagnosed around 60 years of age (4).
IPMNs can macroscopically be categorized in three groups: 10-35% arises in the main pancreatic duct (MD), 40-65% in a branch duct (BD), and 15-40% involves both the main and BDs (mixed type) (39-44). These numbers vary greatly from study to study, but the pattern of duct involvement does guide therapy. For example, examination of resected IPMNs has shown that 62% of MD and 58% of mixed type IPMNs have high-grade dysplasia, and that 44% of MD and 45% of mixed type IPMNs have an associated invasive carcinoma (36). In contrast, only 24% of BD-IPMNs have high-grade dysplasia, and 17% an associated invasive carcinoma (36). Risk assessment and decision-making on which IPMNs to resect and which IPMNs can be safely followed is based on these percentages. However, relying solely on MD vs. BD in decision making can be treacherous as a recent study of 512 IPMNs found that 30% of suspected BD-IPMNs (67/233) had histological involvement of the main pancreatic duct not evident in preoperative imaging (44). Importantly, the misdiagnosed BD-IPMNs had significantly more high-grade dysplasia and were more likely to harbor an associated carcinoma than histologically pure BD-IPMNs (44).
In order to address the complexities of managing patients with an IPMN, consensus guidelines for the management of IPMN and MCN were established in 2012. These guidelines advise that most IPMNs that involve the MD should be surgically resected because of their high rate of malignancy, whereas surgical indications for BD-IPMNs include the presence of “high-risk stigmata” such as mural nodules and symptomatology (Table 2) (36). If there are only so-called “worrisome features” (defined in Table 2), further diagnostic workup is advised. A recent study showed that “high-risk stigmata” had a good correlation with malignancy, but “worrisome features” did not (45). Clearly, development of additional biomarkers that can be used to predict presence of high-grade dysplasia or invasive growth has great potential to improve clinical decision-making (4).
Full table
Histologically, IPMNs can be categorized as gastric-foveolar, intestinal, pancreatobiliary, or oncocytic type based on the direction of differentiation of the neoplastic epithelium as defined by histology and immunolabeling (46,47). In addition, intraductal tubulopapillary neoplasms (ITPN) are recognized as an intraductal neoplasm distinct from IPMNs; however, these lesions are rare, and their precise relationship to the other IPMN subtypes remains to be defined. Most BD-IPMNs have gastric-foveolar histology, whereas intestinal, pancreatobiliary, and oncocytic histologies are seen more often in the main duct type IPMNs. The histologic directions of differentiation in IPMNs have clinical implications and therefore deserve a more detailed discussion.
Gastric-foveolar IPMNs are lined by epithelium resembling foveolar epithelium of the gastric mucosa (Figure 3). The neoplastic epithelial cells have apical mucin with small basally oriented nuclei. The epithelium is usually flat and composed of a single layer of cells, although the neoplastic epithelium can form papillae. Mitoses are rare and most lesions have low-grade dysplasia, although intermediate-/high-grade dysplasia is present in 10% (48). Immunohistochemically the neoplastic epithelium expresses MUC5AC and MUC6, but does not express MUC1 and MUC2 (47). MUC4 is expressed in lesions with higher grades of dysplasia (49). Gastric-foveolar IPMNs can be mixed with pancreatobiliary and intestinal type epithelium. Associated invasive carcinomas are rare, but when present tend to be ductal adenocarcinomas.
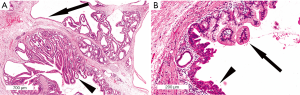
Intestinal IPMNs resemble villous adenomas of the gastrointestinal tract (Figure 4). Long papillae, lined by mucin-secreting neoplastic epithelial cells, protrude from the cyst wall. The neoplastic cells have elongated nuclei and can be pseudostratified. Intestinal IPMNs usually have moderate- to high-grade dysplasia (46). Immunohistochemically the neoplastic cells strongly express MUC2 and MUC5AC, but do not express MUC1. MUC6 is focally expressed in some cases (50). Some also express MUC4 (49). CDX2, a marker of intestinal differentiation, is also expressed in this subtype (47). Associated invasive carcinomas arising from intestinal IPMNs are typically colloid carcinomas (mucinous noncystic adenocarcinomas) with a similar mucin profile (51), but can also be ductal adenocarcinomas or mixed ductal/colloid carcinomas (52).
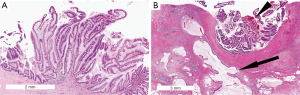
Pancreatobiliary IPMNs are usually high-grade lesions with complex architecture with cribriforming papillae and bridging (Figure 5). The neoplastic cells are cuboidal and have atypical round nuclei with clearly visible nucleoli. Lower-grade dysplasia is rare but when present is characterized by mild atypia with hyperchromasia and enlarged nuclei (46). The neoplastic cells express MUC1, MUC5AC and some also express MUC6. MUC2 is not expressed. Associated invasive carcinomas usually are ductal adenocarcinomas, with the same mucin expression pattern (47,51,53).
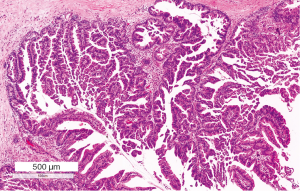
Oncocytic IPMNs, also known as intraductal oncocytic papillary neoplasms (IOPNs), are morphologically the most complex lesions, and have intricate branched papillae with cribriform formations and solid cell nests. They almost always harbor high-grade dysplasia (Figure 6). The cells have abundant eosinophilic cytoplasm, but can have intracellular mucin and intraepithelial mucin pools. MUC1 and MUC6 are expressed by the neoplastic cells. Incidentally goblet cells may be seen expressing MUC2 and MUC5AC. When present, an associated invasive carcinoma is usually the rare oncocytic carcinoma. Although only a small number of cases have been reported this may represent a true subtype based on distinct histology and genetics (54).
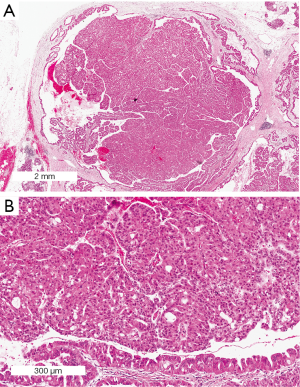
ITPN are the most recently recognized pancreatic intraductal neoplasm and, as mentioned above, may represent a separate entity from IPMN. A predominant tubulopapillary growth of cuboidal neoplastic cells in the affected duct combined with a more solid architecture with minimal cytoplasmic mucin and frequent necrotic foci define this neoplasm (Figure 7). ITPNs often have an overall cribriform appearance. The lesions are always high-grade. MUC6 is expressed in all cells and MUC1 is expressed focally. MUC2 and MUC5AC are negative (55).
IPMN subtypes have been categorized based on their histologic and morphologic features. Although there are clear differences, many IPMNs show mixed histologic features suggesting that these phenotypes do not represent completely distinct underlying pathways. For instance, intestinal and pancreatobiliary IPMNs can both harbor areas with gastric differentiation, and it has been suggested that the low-grade gastric-foveolar type is a common precursor to other types of IPMNs (47).
IPMNs can be a precursor to invasive PDAC. Although IPMNs show many of the genetic alterations involved in PanIN and classic invasive PDAC, such as KRAS, TP53, SMAD4, CDKN2A/P16 (3,6), some genetic alterations, such as activating GNAS mutations and inactivating RNF43 mutations, seem to be more specific for the IPMN precursor pathway (8,9).
Wu et al. sequenced the exomes of eight IPMNs and found that IPMNs contain an average of 26±12 somatic mutations (8). The genes most frequently targeted in IPMNs appear to be KRAS, GNAS, CDKN2A/P16, RNF43, TP53, and SMAD4. In a large follow-up study in which 51 cancer genes were sequenced in 48 IPMNs, Amato and colleagues found that virtually all IPMNs (>90%) harbor a KRAS and/or GNAS mutation, and that CDKN2A/P16, RNF43, TP53, BRAF, and SMAD4 are less commonly targeted (56). KRAS mutations appear to be early events, as close to 90% of low-grade and intermediate-grade IPMNs harbor a KRAS mutation (9). Both intestinal and pancreatobiliary type IPMNs harbor KRAS mutations, while GNAS mutations appear to be more common in intestinal type IPMNs (9,56,57). Of interest, KRAS and BRAF mutations have not been reported in ITPN (58). Interestingly, in vitro research in pancreatic ductal cells found that mutated GNAS may extensively alter gene expression, including expression of mucin genes through the interaction with MAPK and PI3K pathways. Extensively altered expression of MUC2 and MUC5AC in different cell lines suggested a role in morphologic and histologic presentation (59).
As >95% of IPMNs show either a KRAS or GNAS mutation, it is possible that all IPMNs are initiated by a mutation in either one of these genes. Recently an IPMN with associated carcinoma was reported in a patient with McCune-Albright syndrome (post-zygotic noninherited activating GNAS mutations), further establishing the causal role of GNAS in pancreatic tumorigenesis (60).
The targeting of RNF43 tumor suppressor gene in IPMNs is of interest because the protein product of this gene plays an important role in the Wnt/β-catenin pathway (8,56). RNF43 is a transmembrane E3 ligase that down-regulates the Wnt pathway by removing Wnt receptors from the cell surface in intestinal stem cells (61). While further research on the role of RNF43 in IPMN is needed, newer therapies targeting the Wnt/β-catenin pathway may be applicable to IPMN associated invasive PDACs with an RNF43 mutation (62,63).
Another gene that is frequently inactivated in IPMNs is CDKN2A/P16. Homozygous deletions, intragenic mutations coupled with LOH and epigenetic alterations can inactivate CDKN2A/P16 (31). LOH at 9p was seen in 10% of low-grade, 20% of intermediate-grade, and 33% of high-grade IPMNs, and 100% of invasive PDACs (64). Loss of P16 is thus a marker for progression to high-grade dysplasia/invasive carcinoma. A recent study found CDKN2A/P16 mutations in about 5% of IPMNs by NGS, but showed loss of expression in the same tissue in 0% of low-grade, 25% of intermediate-grade, 30% of high-grade, and 50% of invasive IPMNs, suggesting an important role for inactivation by epigenetic mechanisms coupled with LOH (56).
TP53 is mutated late in IPMN progression (56). Mutation of TP53 leads to protein inactivation and typically to the abnormal accumulation of the protein product in the neoplastic cells, reflected by very strong immunostaining for the TP53 protein (Figure 8). Alternatively, completely absent immunostaining indicates a stop codon mutation coupled with LOH (65). TP53 expression is usually normal in low-grade IPMNs, but TP53 expression is altered in a third of intermediate-grade IPMNs and close to half of high-grade IPMNs (66). The tumor suppressor gene SMAD4 is also inactivated late in IPMN progression. Although inactivated in 55% of invasive PDACs, SMAD4 is rarely inactivated in low- or intermediate-grade IPMNs. SMAD4 can be inactivated by homozygous deletion or by intragenic mutations coupled with LOH. Wilentz et al. (29) reported loss of immunohistochemical expression of the SMAD4 protein is a marker for inactivation of the SMAD4 gene (Figure 9). SMAD4 expression was shown to be normal in IPMNs with low-, intermediate- and high-grade dysplasia, while 3 of 4 IPMN associated invasive carcinomas showed loss of SMAD4 (67). Other studies showed similar results, with retained expression of SMAD4 in non-invasive IPMNs but loss of SMAD4 in 3-16% of IPMN associated invasive carcinomas. Iacobuzio-Donahue et al. reported that all 19 colloid carcinomas arising from an IPMN had normal expression of SMAD4, whereas weak staining was seen in 5 of 9 invasive ductal adenocarcinomas arising from an IPMN, suggesting a link of SMAD4 loss with ductal differentiation (68).
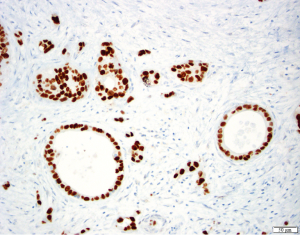
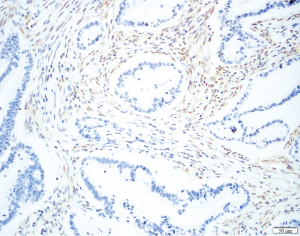
Phosphatidylinositol-3 kinases (PI3K) are lipid-kinases that play a role in proliferation, differentiation, survival, and several other cellular functions. PIK3CA is an oncogene that activates the AKT pathway and is mutated in 10% of intermediate- and high-grade IPMNs, and this genetic targeting of PIK3CA seems to be another late event in the progression of IPMNs (69). PIK3CA was mutated 3 of 11 (37%) ITPNs, which also had overall significantly higher expression of phosphorylated AKT than the control group IPMNs, suggesting that this pathway may be a driver of ITPN development (58). LOH of PTEN, another tumor suppressor gene in the AKT pathway, has been reported in 0% low-grade, 30% intermediate-grade, and 40% high-grade IPMNs. Weak or absent PTEN expression in 30% of IPMNs was also significantly associated with higher nuclear grade, but further studies are needed to evaluate clinical value of PTEN in IPMNs (70). Intriguingly, alterations in the PI3K pathway do not occur commonly in PDACs, pointing to this pathway’s unique importance in IPMNs (5).
STK11, a tumor suppressor gene encoding for the serine threonine protein LKB1, is mutated in the germline of patients with the PJS. PJS is known to cause a 132-fold increase in risk of invasive PDAC and some of these invasive cancers arise from IPMNs (71). Mutations in STK11 are seen in 5% of nonPJS IPMNs (72).
The expression of human telomerase reverse transcriptase (hTERT) and of Sonic hedgehog (Shh) is increased in IPMNs with higher grade of dysplasia, most significantly in progression from intermediate- to high-grade IPMNs (73,74). The loss of expression of the tumor suppressor gene BRG1 has also been association with progression in IPMNs (75). Changes in the expression of some genes in IPMNs are driven by genetic alterations, while in other tumors gene expression changes are produced by epigenetic DNA modifications, microRNAs, post-translational protein modifications, and possible feedback mechanisms. For example, >90% of IPMNs show at least one aberrantly methylated tumor suppressor gene promoter site (76). Genes that have been reported to be methylated in IPMNs included CDKN2A/P16, TP73, APC, hMLH1, MGMT, and E-Cadherin (76). Significantly more genes are methylated in IPMNs with high-grade dysplasia than in IPMNs with low-grade dysplasia. Moreover, some genes may be selectively methylated in high-grade lesions, which may be useful in the clinical management of IPMNs (32,76,77). MicroRNAs are also aberrantly expressed in IPMNs (78). Both MiR-21 and miR-155 are up-regulated in invasive carcinomas associated with IPMNs compared to noninvasive IPMNs and normal tissue, suggesting a role for these microRNAs in carcinogenesis (79,80). These microRNAs regulate key tumor suppressor pathways: miR-21 represses several genes including PTEN (81), and miR-155 represses TP53INP1 (80). Downregulation of microRNA MiR-101 has also been shown in progression of IPMNs. MiR-101 can silence EZH2 expression in IPMN (82), and in IPMNs EZH2 expression has an inverse correlation in expression with the tumor suppressor CDKN1B/p27. EZH2 might transcriptionally silence CDKN1B/p27 and is also known to methylate the protein histone 3 at lysine 27 (83).
It has also been suggested that the pattern of expression of certain microRNAs can be used as a marker of different IPMN subtypes. MiR-196a expression is associated with intestinal IPMN (84) and miR-200c, miR-141, miR-216 could be used to mark dysplastic progression in IPMN-tissue (85), and cyst fluid (86). MicroRNAs can also be detected in serum and can have discriminating diagnostic applications (87). Other diagnostic approaches for early diagnosis of high-grade/invasive IPMN may be detection of TP53 and/or SMAD4 mutations in pancreatic juice or cyst fluid (88), circulating tumor cells (89), mRNA binding proteins (90), ubiquitin and thymosin-β4 in EUS FNA (91), and monoclonal antibodies (92).
Thus, a number of genetic, histological and clinical studies have defined the molecular basis for the development of IPMNs which in turn suggests novel molecular biomarkers and novel therapeutic approaches for these neoplasms.
Mucinous cystic neoplasm (MCN)
MCN is the least common of the precursor lesions that can give rise to invasive PDAC. MCNs occur almost exclusively women and usually in the tail of the pancreas. MCNs are cyst forming neoplasms, and characteristically the cysts do not communicate with the pancreatic duct system. By definition, MCNs contain a characteristic ovarian-type stroma (Figure 10). One theory on the pathogenesis of MCNs argues that they are the result of ectopic gonadal mesenchyme that is incorporated in the pancreas during the fourth and fifth weeks of embryogenesis as a result of the close proximity of the left primordial gonad to the dorsal pancreatic anlage which gives rise to the pancreatic body and tail. This could also explain MCNs at the contralateral side in the hepatobiliary tract (93,94). However, because this cannot explain the rare occurrence of MCN in male patients, an alternative theory has been put forth which suggests that neoplastic epithelial cells of MCNs induce ovarian stromal differentiation in cells that are normally present in the pancreas (95).
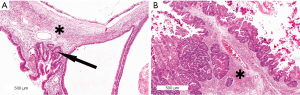
MCNs account for approximately 8% of all resected cystic lesions of the pancreas (96,97). Small MCNs (<3 cm) are usually incidental findings, whereas larger MCNs may produce nonspecific complaints such as abdominal discomfort and the sensation of a mass in the epigastric region. Surgical resection is recommended for all surgically fit patients (Table 2). Up to one-third of resected MCNs have an associated invasive carcinoma, although more recent studies report lower percentages (5-15%), likely due to the fact that smaller low-grade MCNs are being detected incidentally in patients imaged for other reasons (98-100). Invasive adenocarcinomas arising in MCNs usually resemble a common PDAC but can also have a mucinous histology. Because invasive carcinoma can arise very focally in an MCN, when MCNs are resected they should be sampled extensively, if not completely, by the examining pathologist (99). Patients with a surgically resected noninvasive MCN are cured after the resection. The 5-year survival rate for patients with an MCN with an associated invasive carcinoma is about 50-60%, depending on the extent of invasion (96).
Grossly, in contrast to IPMNs, MCNs do not communicate with the pancreatic ductal system. Most MCNs form large (average size 10 cm) multilocular lesions containing thick mucin, or sometimes mucin tinged by hemorrhage. Lesions with low-grade dysplasia usually have a smooth and glistering internal surface, whereas lesions with high-grade dysplasia are lined by epithelium with papillary projections. MCNs with an associated invasive carcinoma are often large and multilocular and contain papillary projections or mural nodules (96,101).
Microscopically, the cysts of MCNs are lined by a columnar mucin-producing neoplastic epithelium. By definition, they also have an ovarian-type stroma consisting of densely packed spindle cells with round to elongated nuclei and a small amount of cytoplasm (Figure 10). The stromal cells express inhibin, estrogen and progesterone receptors, as well as vimentin, smooth-muscle actin, and desmin. In some lesions the stroma may become fibrotic and hypocellular and be more difficult to recognize. The epithelial lining of the cysts consists of mucin-producing tall columnar epithelial cells with pseudopyloric, gastric-foveolar, small-intestinal or large-intestinal differentiation. Squamous differentiation is only rarely seen (95). The epithelial cells express cytokeratins 7, 8, 18, and 19, the gastric type mucin MUC5A, and pancreatic type mucin DUPAN-2 and CA19-9, whereas scattered goblet-like cells express the intestinal MUC2. MUC1 expression is observed in most ductal adenocarcinoma arising from MCN, but is negative in the associated noninvasive components (102). The degree of dysplasia in MCN can vary greatly and change abruptly from minimal to severe or even focal invasive growth. The highest degree of dysplasia present in an MCN determines the classification of the lesions as MCN with low-grade, intermediate-grade, or high-grade dysplasia (95). The vast majority (70-80%) of MCNs are low-grade (98,100).
MCNs is less well-characterized at the genetic level than are PanINs and IPMNs. However, recent whole-exome sequencing of carefully microdissected MCNs has revealed that the neoplastic epithelium has an average of 16±7.6 somatic mutations and relatively few allelic losses (8). KRAS is the most frequently mutated gene in MCN. Using Sanger sequencing KRAS mutations have been found in 25% (7/27) of MCNs with low-grade dysplasia, 40% (5/13) of MCNs with intermediate-grade dysplasia, and 90% (8/9) of MCNs with high-grade dysplasia or invasive carcinoma. Mutations in TP53 are a relatively late event occurring only in areas with high-grade dysplasia or an associated invasive carcinoma (103). Whole-exome sequencing identified RNF43 mutations in 3 of the 8 MCNs examined (8). Loss of SMAD4 is a late event in neoplastic progression of MCN and found in associated invasive adenocarcinomas but not typically in noninvasive components of MCNs (104). Rarely PIK3CA gene mutations are found in MCN, but these seem confined to those with high-grade dysplasia (105). Hypermethylation of P14 and P16 has been reported in about 15% of non-invasive MCNs (106).
Global gene expression profiling identified a number of genes that are up-regulated in the epithelium of MCNs, including S100, PSCA, C-MYC, STK6/STK15, cathepsin E, TCF4, and pepsinogen C. In addition, activation of the Notch pathway was shown in the epithelial component by the demonstration of overexpression of Jagged1 and the downstream Notch pathway member Hes1. Overexpression of steroidogenic acute regulatory (STAR) protein and estrogen receptor 1 (ESR1) occurs in the stroma (107).
Conclusions
PDAC is a deadly disease. The key to reducing deaths from PDAC is to detect pancreatic neoplasia at a very early and still curable stage or, even better, to detect and treat precursor lesions before they transform into incurable invasive cancers. PDAC develops from several histologically and genetically distinct precursor lesions providing an opportunity for early detection and prevention (2). Moreover, genetic studies have suggested that the window of opportunity to diagnose and treat a precursor lesions is almost 12 years (10).
While the genes that are recurrently mutated in PDAC and in precursor lesions (such as KRAS, GNAS, TP53, CDKN2A/P16, SMAD4) are prime targets for early detection efforts, some of the genes that are less commonly mutated (such as ATM, BRCA2, and RNF43) are potentially more therapeutically targetable. Moreover, therapeutic targeting of one or more core signaling pathways involved in PDAC instead of a specific genetic alteration may be important to circumvent genetic heterogeneity of PDAC.
Although progress in the therapy of patients with PDAC is invaluable, early detection and prevention of PDAC are likely to be more effective to decrease mortality (2). Today biomarkers can be assessed in cyst fluid aspirated by fine needle aspiration or in secreted pancreatic juice collected in the duodenum (9,88,108,109). Recent studies revealed genetic alterations in pancreatic cyst fluid that can discriminate between a completely harmless cyst such as serous cyst adenoma and a premalignant cyst such as IPMN and MCN (8). However, the ultimate goal is to identify those patients with a high-grade precursor lesion and/or early invasive PDAC (88). Those patients would benefit from a surgical resection, whereas patients with lesions with only low-grade dysplasia could be safely followed without surgery. Such definitive biomarkers are not yet available, but further dissection of the genetic progression of PDAC precursor lesions will hopefully lead to the identification of biomarkers that indicate high-grade dysplasia or transition to invasive growth. Ultimately this will lead to better risk stratification of patients with pancreatic cancer precursor lesions and patients at increased risk of PDAC. Newer gene-based tests have the potential to greatly aid in clinical decision-making and the selection of patients who would benefit from surgical treatment, while on the other hand patients with low-risk lesions could be spared from an operation.
Acknowledgements
We thank Dr. Jeanin van Hooft for generously providing the Endoscopic picture of a bulging ampulla of Vater with extruding thick mucin in a patient with an IPMN.
Funding: This work was supported by the Union for International Cancer Control (UICC) Yamagiwa-Yoshida Memorial International Cancer Study Grants; the Nijbakker-Morra Stichting; the Lisa Waller Hayes Foundation; Dutch Digestive Foundation (MLDS CDG 14-02); Dutch Cancer Society; the Kaya Tuncer Career Development Award in GI Cancer Prevention; the AGA-Bernard Lee Schwartz Research Scholar Award in Pancreatic Cancer; Sigma Beta Sorority; Joseph C. Monastra Foundation; Rolfe Pancreatic Cancer Foundation; The Sol Goldman Pancreatic Cancer Research Center; and NIH grant CA62924.
Disclosure: Dr. Hruban receives royalty payments from Myriad Genetics from the PalB2 invention. This is managed by Johns Hopkins University and has been disclosed. The other authors declare no conflict of interest.
References
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [PubMed]
- Hruban RH, Takaori K, Canto M, et al. Clinical importance of precursor lesions in the pancreas. J Hepatobiliary Pancreat Surg 2007;14:255-63. [PubMed]
- Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res 2000;6:2969-72. [PubMed]
- Matthaei H, Schulick RD, Hruban RH, et al. Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol 2011;8:141-50. [PubMed]
- Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012;491:399-405. [PubMed]
- Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801-6. [PubMed]
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114-7. [PubMed]
- Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011;108:21188-93. [PubMed]
- Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66. [PubMed]
- Iacobuzio-Donahue CA. Genetic evolution of pancreatic cancer: lessons learnt from the pancreatic cancer genome sequencing project. Gut 2012;61:1085-94. [PubMed]
- Yachida S, Iacobuzio-Donahue CA. Evolution and dynamics of pancreatic cancer progression. Oncogene 2013;32:5253-60. [PubMed]
- Iacobuzio-Donahue CA, van der Heijden MS, Baumgartner MR, et al. Large-scale allelotype of pancreaticobiliary carcinoma provides quantitative estimates of genome-wide allelic loss. Cancer Res 2004;64:871-5. [PubMed]
- Kowalski J, Morsberger LA, Blackford A, et al. Chromosomal abnormalities of adenocarcinoma of the pancreas: identifying early and late changes. Cancer Genet Cytogenet 2007;178:26-35. [PubMed]
- Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 2009;324:217. [PubMed]
- Roberts NJ, Jiao Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2012;2:41-6. [PubMed]
- Zhen DB, Rabe KG, Gallinger S, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med 2014. [Epub ahead of print]. [PubMed]
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006;12:3209-15. [PubMed]
- Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009;302:1790-5. [PubMed]
- Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2004;28:977-87. [PubMed]
- Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol 2006;30:1067-76. [PubMed]
- Konstantinidis IT, Vinuela EF, Tang LH, et al. Incidentally discovered pancreatic intraepithelial neoplasia: what is its clinical significance? Ann Surg Oncol 2013;20:3643-7. [PubMed]
- Shi C, Klein AP, Goggins M, et al. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin Cancer Res 2009;15:7737-43. [PubMed]
- van Heek NT, Meeker AK, Kern SE, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol 2002;161:1541-7. [PubMed]
- Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012;142:730-733.e9.
- Löhr M, Klöppel G, Maisonneuve P, et al. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia 2005;7:17-23. [PubMed]
- Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res 2006;66:95-106. [PubMed]
- Matthaei H, Wu J, Dal Molin M, et al. GNAS sequencing identifies IPMN-specific mutations in a subgroup of diminutive pancreatic cysts referred to as "incipient IPMNs". Am J Surg Pathol 2014;38:360-3. [PubMed]
- Wilentz RE, Geradts J, Maynard R, et al. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res 1998;58:4740-4. [PubMed]
- Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2000;60:2002-6. [PubMed]
- Lüttges J, Galehdari H, Bröcker V, et al. Allelic loss is often the first hit in the biallelic inactivation of the p53 and DPC4 genes during pancreatic carcinogenesis. Am J Pathol 2001;158:1677-83. [PubMed]
- Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res 1997;57:3126-30. [PubMed]
- Sato N, Ueki T, Fukushima N, et al. Aberrant methylation of CpG islands in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology 2002;123:365-72. [PubMed]
- Murphy SJ, Hart SN, Lima JF, et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology 2013;145:1098-1109.e1.
- Matthaei H, Hong SM, Mayo SC, et al. Presence of pancreatic intraepithelial neoplasia in the pancreatic transection margin does not influence outcome in patients with R0 resected pancreatic cancer. Ann Surg Oncol 2011;18:3493-9. [PubMed]
- Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med 2004;351:1218-26. [PubMed]
- Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183-97. [PubMed]
- de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010;8:806-11. [PubMed]
- Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 2008;191:802-7. [PubMed]
- Schnelldorfer T, Sarr MG, Nagorney DM, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg 2008;143:639-46; discussion 646. [PubMed]
- Shimizu Y, Yamaue H, Maguchi H, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas 2013;42:883-8. [PubMed]
- Furukawa T, Hatori T, Fujita I, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut 2011;60:509-16. [PubMed]
- Lafemina J, Katabi N, Klimstra D, et al. Malignant progression in IPMN: a cohort analysis of patients initially selected for resection or observation. Ann Surg Oncol 2013;20:440-7. [PubMed]
- Kang MJ, Lee KB, Jang JY, et al. Evaluation of clinical meaning of histological subtypes of intraductal papillary mucinous neoplasm of the pancreas. Pancreas 2013;42:959-66. [PubMed]
- Fritz S, Klauss M, Bergmann F, et al. Pancreatic main-duct involvement in branch-duct IPMNs: an underestimated risk. Ann Surg 2014;260:848-55; discussion 855-6. [PubMed]
- Aso T, Ohtsuka T, Matsunaga T, et al. "High-risk stigmata" of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2014;43:1239-43. [PubMed]
- Furukawa T, Klöppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 2005;447:794-9. [PubMed]
- Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an "intestinal" pathway of carcinogenesis in the pancreas. Am J Surg Pathol 2004;28:839-48. [PubMed]
- Ban S, Naitoh Y, Mino-Kenudson M, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol 2006;30:1561-9. [PubMed]
- Kitazono I, Higashi M, Kitamoto S, et al. Expression of MUC4 mucin is observed mainly in the intestinal type of intraductal papillary mucinous neoplasm of the pancreas. Pancreas 2013;42:1120-8. [PubMed]
- Basturk O, Khayyata S, Klimstra DS, et al. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol 2010;34:364-70. [PubMed]
- Adsay NV, Merati K, Nassar H, et al. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: Coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol 2003;27:571-8. [PubMed]
- Nakamura A, Horinouchi M, Goto M, et al. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol 2002;197:201-10. [PubMed]
- Terada T, Ohta T, Sasaki M, et al. Expression of MUC apomucins in normal pancreas and pancreatic tumours. J Pathol 1996;180:160-5. [PubMed]
- Liszka L, Pajak J, Zielińska-Pajak E, et al. Intraductal oncocytic papillary neoplasms of the pancreas and bile ducts: a description of five new cases and review based on a systematic survey of the literature. J Hepatobiliary Pancreat Sci 2010;17:246-61. [PubMed]
- Yamaguchi H, Shimizu M, Ban S, et al. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2009;33:1164-72. [PubMed]
- Amato E, Molin MD, Mafficini A, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 2014;233:217-27. [PubMed]
- Dal Molin M, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol 2013;20:3802-8. [PubMed]
- Yamaguchi H, Kuboki Y, Hatori T, et al. Somatic mutations in PIK3CA and activation of AKT in intraductal tubulopapillary neoplasms of the pancreas. Am J Surg Pathol 2011;35:1812-7. [PubMed]
- Komatsu H, Tanji E, Sakata N, et al. A GNAS mutation found in pancreatic intraductal papillary mucinous neoplasms induces drastic alterations of gene expression profiles with upregulation of mucin genes. PLoS One 2014;9:e87875. [PubMed]
- Parvanescu A, Cros J, Ronot M, et al. Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms:: GNAS-activating mutations in pancreatic carcinogenesis. JAMA Surg 2014;149:858-62. [PubMed]
- de Lau W, Peng WC, Gros P, et al. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev 2014;28:305-16. [PubMed]
- Wall I, Schmidt-Wolf IG. Effect of Wnt inhibitors in pancreatic cancer. Anticancer Res 2014;34:5375-80. [PubMed]
- Jiang X, Hao HX, Growney JD, et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A 2013;110:12649-54. [PubMed]
- Wada K. p16 and p53 gene alterations and accumulations in the malignant evolution of intraductal papillary-mucinous tumors of the pancreas. J Hepatobiliary Pancreat Surg 2002;9:76-85. [PubMed]
- Baas IO, Hruban RH, Offerhaus GJ. Clinical applications of detecting dysfunctional p53 tumor suppressor protein. Histol Histopathol 1999;14:279-84. [PubMed]
- Abe K, Suda K, Arakawa A, et al. Different patterns of p16INK4A and p53 protein expressions in intraductal papillary-mucinous neoplasms and pancreatic intraepithelial neoplasia. Pancreas 2007;34:85-91. [PubMed]
- Biankin AV, Biankin SA, Kench JG, et al. Aberrant p16(INK4A) and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut 2002;50:861-8. [PubMed]
- Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, et al. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol 2000;157:755-61. [PubMed]
- Schönleben F, Qiu W, Remotti HE, et al. PIK3CA, KRAS, and BRAF mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/C) of the pancreas. Langenbecks Arch Surg 2008;393:289-96. [PubMed]
- Garcia-Carracedo D, Turk AT, Fine SA, et al. Loss of PTEN expression is associated with poor prognosis in patients with intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res 2013;19:6830-41. [PubMed]
- Brosens LA, van Hattem WA, Jansen M, et al. Gastrointestinal polyposis syndromes. Curr Mol Med 2007;7:29-46. [PubMed]
- Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol 2001;159:2017-22. [PubMed]
- Hashimoto Y, Murakami Y, Uemura K, et al. Telomere shortening and telomerase expression during multistage carcinogenesis of intraductal papillary mucinous neoplasms of the pancreas. J Gastrointest Surg 2008;12:17-28; discussion 28-9. [PubMed]
- Jang KT, Lee KT, Lee JG, et al. Immunohistochemical expression of Sonic hedgehog in intraductal papillary mucinous tumor of the pancreas. Appl Immunohistochem Mol Morphol 2007;15:294-8. [PubMed]
- Dal Molin M, Hong SM, Hebbar S, et al. Loss of expression of the SWI/SNF chromatin remodeling subunit BRG1/SMARCA4 is frequently observed in intraductal papillary mucinous neoplasms of the pancreas. Hum Pathol 2012;43:585-91. [PubMed]
- House MG, Guo M, Iacobuzio-Donahue C, et al. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis 2003;24:193-8. [PubMed]
- Hong SM, Omura N, Vincent A, et al. Genome-wide CpG island profiling of intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res 2012;18:700-12. [PubMed]
- Lubezky N, Loewenstein S, Ben-Haim M, et al. MicroRNA expression signatures in intraductal papillary mucinous neoplasm of the pancreas. Surgery 2013;153:663-72. [PubMed]
- Caponi S, Funel N, Frampton AE, et al. The good, the bad and the ugly: a tale of miR-101, miR-21 and miR-155 in pancreatic intraductal papillary mucinous neoplasms. Ann Oncol 2013;24:734-41. [PubMed]
- Gironella M, Seux M, Xie MJ, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A 2007;104:16170-5. [PubMed]
- Meng F, Henson R, Wehbe-Janek H, et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007;133:647-58. [PubMed]
- Nakahara O, Takamori H, Iwatsuki M, et al. Carcinogenesis of intraductal papillary mucinous neoplasm of the pancreas: loss of microRNA-101 promotes overexpression of histone methyltransferase EZH2. Ann Surg Oncol 2012;19 Suppl 3:S565-71. [PubMed]
- Kuroki H, Hayashi H, Okabe H, et al. EZH2 is associated with malignant behavior in pancreatic IPMN via p27Kip1 downregulation. PLoS One 2014;9:e100904. [PubMed]
- Aso T, Ohtsuka T, Tamura K, et al. Elevated expression level of microRNA-196a is predictive of intestinal-type intraductal papillary mucinous neoplasm of the pancreas. Pancreas 2014;43:361-6. [PubMed]
- Lahat G, Lubezky N, Loewenstein S, et al. Epithelial-to-mesenchymal transition (EMT) in intraductal papillary mucinous neoplasm (IPMN) is associated with high tumor grade and adverse outcomes. Ann Surg Oncol 2014;21 Suppl 4:S750-7. [PubMed]
- Wang J, Paris PL, Chen J, et al. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett 2015;356:404-9. [PubMed]
- Abue M, Yokoyama M, Shibuya R, et al. Circulating miR-483-3p and miR-21 is highly expressed in plasma of pancreatic cancer. Int J Oncol 2015;46:539-47. [PubMed]
- Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 2013;11:719-30.e5.
- Rhim AD, Thege FI, Santana SM, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 2014;146:647-51. [PubMed]
- Morimatsu K, Aishima S, Yamamoto H, et al. Insulin-like growth factor II messenger RNA-binding protein-3 is a valuable diagnostic and prognostic marker of intraductal papillary mucinous neoplasm. Hum Pathol 2013;44:1714-21. [PubMed]
- Rebours V, Le Faouder J, Laouirem S, et al. In situ proteomic analysis by MALDI imaging identifies ubiquitin and thymosin-β4 as markers of malignant intraductal pancreatic mucinous neoplasms. Pancreatology 2014;14:117-24. [PubMed]
- Das KK, Xiao H, Geng X, et al. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN). Gut 2014;63:1626-34. [PubMed]
- Erdogan D, Lamers WH, Offerhaus GJ, et al. Cystadenomas with ovarian stroma in liver and pancreas: an evolving concept. Dig Surg 2006;23:186-91. [PubMed]
- Erdogan D, Kloek J, Lamers WH, et al. Mucinous cystadenomas in liver: management and origin. Dig Surg 2010;27:19-23. [PubMed]
- Zamboni G, Fukushima N, Hruban RH, et al. Mucinous cystic neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al, editors. WHO Classification of tumors of the digestive system. 4th ed. Lyon: IARC, 2010:300-3.
- Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 1999;23:410-22. [PubMed]
- Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch 2004;445:168-78. [PubMed]
- Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas 2011;40:67-71. [PubMed]
- Wilentz RE, Albores-Saavedra J, Zahurak M, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol 1999;23:1320-7. [PubMed]
- Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg 2008;247:571-9. [PubMed]
- Fukushima N, Fukayama M. Mucinous cystic neoplasms of the pancreas: pathology and molecular genetics. J Hepatobiliary Pancreat Surg 2007;14:238-42. [PubMed]
- Lüttges J, Feyerabend B, Buchelt T, et al. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol 2002;26:466-71. [PubMed]
- Jimenez RE, Warshaw AL, Z’graggen K, et al. Sequential accumulation of K-ras mutations and p53 overexpression in the progression of pancreatic mucinous cystic neoplasms to malignancy. Ann Surg 1999;230:501-9; discussion 509-11. [PubMed]
- Iacobuzio-Donahue CA, Wilentz RE, Argani P, et al. Dpc4 protein in mucinous cystic neoplasms of the pancreas: frequent loss of expression in invasive carcinomas suggests a role in genetic progression. Am J Surg Pathol 2000;24:1544-8. [PubMed]
- Garcia-Carracedo D, Chen ZM, Qiu W, et al. PIK3CA mutations in mucinous cystic neoplasms of the pancreas. Pancreas 2014;43:245-9. [PubMed]
- Kim SG, Wu TT, Lee JH, et al. Comparison of epigenetic and genetic alterations in mucinous cystic neoplasm and serous microcystic adenoma of pancreas. Mod Pathol 2003;16:1086-94. [PubMed]
- Fukushima N, Sato N, Prasad N, et al. Characterization of gene expression in mucinous cystic neoplasms of the pancreas using oligonucleotide microarrays. Oncogene 2004;23:9042-51. [PubMed]
- Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut 2013;62:1024-33. [PubMed]
- Sadakari Y, Kanda M, Maitani K, et al. Mutant KRAS and GNAS DNA Concentrations in Secretin-Stimulated Pancreatic Fluid Collected from the Pancreatic Duct and the Duodenal Lumen. Clin Transl Gastroenterol 2014;5:e62. [PubMed]