
Investigating disparities: the effect of social environment on pancreatic cancer survival in metastatic patients
Introduction
Pancreatic adenocarcinoma (PCA) has a poor prognosis, with an average 5-year survival rate of 8.5% (1) and a median survival after diagnosis of metastatic disease of about 12 months (1). The poor outcomes of PCA can be partially explained by the difficulty of detecting the disease early, leading to an often-advanced clinical presentation. Health disparities may also affect disease outcome, and prognosis.
Incidence rates of PCA are over 50% higher in African-American patients compared to rates in White patients or any other racial group (2). Several studies have demonstrated decreased survival among African American and Hispanic patients with PCA (3-5). Beyond race/ethnicity, other social determinants of health such as socio-economic status (SES) (i.e., low education, employment, income) also have been identified as independent, negative prognostic variables for PCA survival (4-6). Patients with higher SES were found to be more likely to undergo surgery, chemotherapy and radiation treatments, which improve outcomes in PCA (5,6). Further, investigations into the interaction between race and SES have suggested that the observed survival difference between racial groups can be partly accounted for by treatment disparities and variation in a patient’s SES (5).
Macro-environmental factors including neighborhood socioeconomic status (nSES) can also contribute to cancer disparities. nSES is often defined by U.S. Census in cancer studies as variables related to SES that describe the economic (e.g., employment, income, poverty), physical (e.g., housing/transportation structure), and social (e.g., education, immigration/migration) characteristics of a person’s place of residence (7). There are at least two hypotheses that may explain how nSES can impact cancer outcomes. First, under a chronic stress hypothesis, residents from disadvantaged neighborhoods could experience greater emotional stress and constant “wear and tear” on the body that can affect cancer initiation and progression (8). Second, low nSES may correlate with factors related to health care access, particularly access to quality treatment (9).
In PCA, a recent population-based study showed that nSES remained significantly associated with PCA survival, even when adjusted for patient-level variables, including age, race, insurance and marital status (10,11). However, this study and other similar studies are limited by a lack of information regarding well-known patient clinical comorbidities (i.e., smoking, diabetes), which can have an effect on PCA outcomes (12,13). Further, the majority of prior SES studies in PCA focused primarily on early-stage disease when most patients are diagnosed with advanced disease (4,12,13). Thus, the relationship between nSES and metastatic disease remains uncharacterized. Therefore, the goal of this study was to investigate whether nSES measures are associated with survival in a population of metastatic pancreatic adenocarcinoma (mPCA) patients who were treated at a tertiary cancer center and have both detailed health record and nSES data. Additionally, this study sought to determine whether nSES variables can improve prediction of mPCA survival models, which typically only consider patient-level data. We present the following article in accordance with the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jgo-20-39.
Methods
Study population
Retrospective collection of patient data was approved by Fox Chase Cancer Center IRB (IRB #11-815). A waiver of informed consent was obtained from the IRB due to the study’s retrospective design.
With IRB approval, patients diagnosed with mPCA at Fox Chase Cancer Center (Philadelphia, PA), a tertiary care center, between 2010 and 2016 were retrospectively identified using the institution’s tumor registry (IRB #11-815). Cases included patients who presented with metastatic disease, as well as patients who presented with earlier stage disease and later recurred. Cases with incomplete medical record data, missing address information, or with a histologic diagnosis other than PCA were excluded, resulting in a total study population of 370 patients. The primary clinical outcome of interest was overall survival from date of diagnosis of metastatic disease. Information regarding date of death was obtained through the tumor registry. Patients who were living as of December 31, 2016 or date of last follow-up were considered censored.
Patient variables
Each patient chart was examined and quality control checked by two independent reviewers to ensure accuracy of the data. Risk factors previously found to be associated with pancreatic cancer risk or survival were included in this analysis: age (1), sex (1), race (1,5), body mass index (BMI) (14), diabetes (15) (yes/no), statin use (16) (yes/no), tobacco use (17) (yes/no), self-identified Jewish ancestry (18), family history of PCA (18), and marital status (10) (married, not married). Stage at diagnosis was documented, in addition to the received treatment modalities during the disease course (radiation, chemotherapy, and surgical resection prior to diagnosis of metastatic disease). We included chemotherapy administered at or after the time of metastatic diagnosis regardless of regimen or number of drugs used. In addition, radiation therapies were directed at the tumor, rather than palliative treatment for metastases. Age-adjusted Charlson Comorbidity Index (CCI) was calculated for each patient by utilizing the published scoring method (19).
nSES variables
Our study looked at nSES variables that have been found to be associated with outcomes related to cancer and other health conditions (11,20-23). Variables of interest included: (I) deprivation (24), (II) racial concentration (25), (III) transportation access (the % of residents owning a vehicle), (IV) immigration (the % of foreign born residents), and (V) neighborhood stability (the % residents living in the same house as one year prior). Deprivation was measured using a previously validated composite SES measure, created by principal component analysis of seven indicator variables: education (out of individuals age 25 and older, proportion with college, high school and less than high school weighted by 16, 12 and 9 respectively), proportion with a blue collar job, proportion older than age 16 in the workforce without a job, median household income, percent below 200% of the poverty line, median rent, median house value. Deprivation scores for the state of PA ranged from −4.36 (negative scores indicate high deprivation) to 4.99 (positive scores indicate low deprivation). The index is categorized into quintiles based on the census tract values for the overall state with 1 being the highest level of deprivation and 5 being the lowest level of deprivation. Racial concentration (RC) was defined as the degree of isolation/separation of racial/ethnic groups in a neighborhood, with a standard score ranging from −1 [concentration of Non-Hispanic Blacks (NHB)] to 1 [concentration of Non-Hispanic Whites (NHW)]. This measure was categorized into quartiles, based on prior literature (11). Transportation access, immigration, and stability were analyzed as continuous variables.
nSES variables were derived from the U.S. Census American Community Survey (ACS) collected at the census tract level between 2011 and 2015. Census tracts are geographic subdivisions of a county used for the purpose of government population tracking, with an average of 4,000 residents residing in each tract (26). Residential addresses of mPCA patients were geocoded up to the census tract level and assigned a Federal Information Processing Standard (FIPS) geocode (27,28) at the census tract level using Arc GIS software v. 10.6. (ESRI; Redlands, CA). Patient information was then linked to the nSES variables from the U.S. census mentioned above via the FIPS code using Stata v. 11.0 (College Station, TX). Thus, patients residing in the same census tract were assumed to have the same neighborhood characteristics. There were 312 unique census tracts included in this analysis.
Statistical analysis
The relationship between survival, patient-level variables and nSES variables were assessed in multivariable models with and without nSES variables via mixed effect Cox proportional hazards regression models. We accounted for potential clustering effects of individuals within the same CT using random effects (29,30). Hazards ratios and 95% confidence intervals (95% CI) are presented. Variables with P values <0.05 were considered significant. To preliminarily assess the potential clinical relevance of nSES variables, multivariable models with patient-only variables were compared to multivariable models with both patient and nSES variables using likelihood ratio tests (31), time-varying receiver operating characteristic (ROC) curves estimates (32), and the Akaike Information Criteria (AIC) (33).
Results
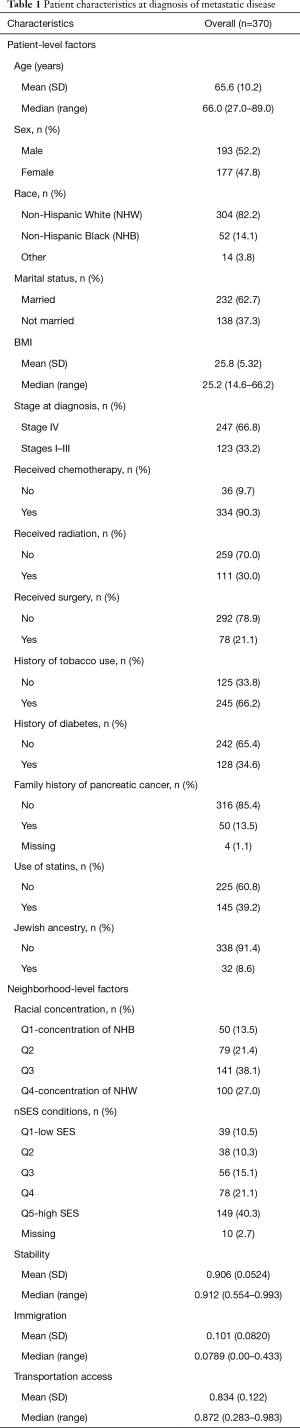
The study population included 370 patients with mPCA. Table 1 shows a summary of both patient-level and nSES variables that were considered in the analysis. Patient diagnosis age ranged from 27–89 years (mean 65.6 years), slightly more than half of the patient population was male, and about 80% were NHW. Median survival was 8 months (average follow-up time for all cases =10 months) after metastatic diagnosis with 90% of patients deceased by the end of the study period (December 31, 2016). Most individuals were diagnosed with metastatic (stage IV) disease, and 90% of patients received chemotherapy treatments. About two-thirds of patients had a history of tobacco use, 63% were married, 35% had a history of diabetes, 39% used statins, 14% had a family history of PCA, and 9% had Jewish ancestry.
Full table
Sixty-one percent of patients lived in areas with low deprivation. Approximately half of patients in the study lived in neighborhoods which were categorized as high stability, low % immigration, and high transportation access (i.e., neighborhoods were above the median for these variables). For residential concentration, over a quarter of patients lived in neighborhoods with high concentration of NHW and 14% lived in areas with high concentration of NHB.
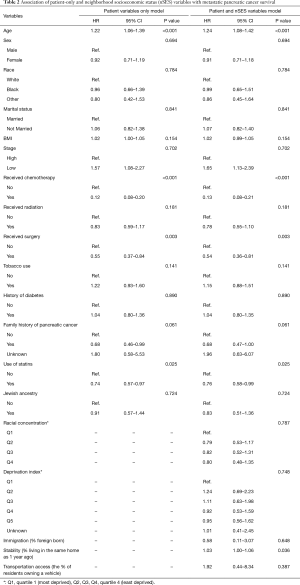
Referring to Table 2, patient-level variables that were found to be significant predictors of survival were consistent across multivariable models. Increasing age was associated with an increased hazard of death [HR =1.24 (95% CI: 1.08–1.42); P<0.001]. The following patient variables demonstrated inverse relationship with risk of death including: chemotherapy treatment [HR =0.13 (95% CI: 0.08–0.21); P<0.001], initial surgical resection [HR =0.54 (95% CI: 0.36–0.81); P=0.003], and statin use [HR =0.76 (95% CI: 0.58–0.99); P=0.025]. In the multivariable model with both patient and nSES measures, only the nSES measure of stability (the % residents living in the same house as one year prior) was statistically significant [HR =1.03 (95% CI: 1.001–1.06); P=0.036]. The remaining nSES variables, which included deprivation index (P=0.784), transportation access (P=0.387), racial concentration (P=0.787) and immigration (P=0.648), were not statistically significant.
Full table
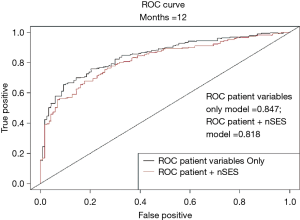
To preliminary assess the potential clinical relevance of nSES measures, we compared a multivariable model with patient-level variables only to a multivariable model with both patient and a nSES variable (neighborhood stability). After the addition of neighborhood stability, a decrease in AIC was observed, indicative of improved model fit [AIC patient variable only multivariable model (71.51) vs. AIC with nSES (50.03)]. In addition, the likelihood ratio test showed that this improvement was statistically significant (P=0.002). However, referring to Figure 1, the area under the ROC curve at 12 months decreased when including the nSES with the significant patient-level variables, indicating that including neighborhood stability does not help to improve prediction of mPCA survival.
Discussion
Health disparities in pancreatic cancer is an understudied area. While studies comparing PCA disease rates by race/ethnicity (2,4) have been conducted, very few also incorporate or study the role of other determinants of disparity such as the effect of nSES. This is the first study to investigate the effect of nSES on pancreatic cancer survival in a group of metastatic patients. Our study found that patients diagnosed with mPCA between 2010–2016 have a median survival of 8 months, which is consistent with the national average of 8–11 months (1,34). We found that while neighborhood stability appeared significantly related to shorter mPCA survival, the addition of this variable did not improve prediction of mPCA survival more than patient variables.
The inverse association between neighborhood stability and PCA survival was not in the expected direction. Previous studies have suggested that neighborhood stability may contribute to stronger social networks and lower perceived stress that can lead to improved health outcomes (35-37). Our results demonstrated an association between high neighborhood stability and decreased survival in mPCA patients. While the direction of this relationship generally does not follow the hypotheses put forth in previous studies (36,37), one study did find an association between residential stability and increased odds of diabetes, a risk factor for pancreatic cancer (23). Thus, while it is possible that this nSES finding could be a spurious association, there still remains a lack of empiric evidence linking neighborhood stability to cancer outcomes specifically. Further, the majority of patients in our study come from high nSES environments and it’s likely that the lack of nSES variation in our sample (i.e., limited heterogeneity) would make it insufficient to detect possible associations between nSES and mPCA. Given the finding of a significant association between nSES and mPCA survival in our relatively homogenous sample suggests that future investigations in larger, more socioeconomically diverse study populations are warranted.
Our standard model demonstrated several patient-level variables as significant predictors of survival. These variables included age, receipt of chemotherapy, and initial surgical resection of the primary tumor. Our findings are consistent with the literature, in which the effect on survival by these factors has been well established (5,6,38-41). This result provides validation to the survival model in this study. One relatively novel patient-level finding in this study was the significant effect of statin use on PCA survival. This finding supports available literature which suggests that statins may slow tumor development and progression by disrupting expression and activity of downstream proteins involved in cell signaling and growth (42-44). Epidemiologic studies have also demonstrated an inverse relationship between statin use and pancreatic cancer risk (16,45). Additionally, there is some evidence that statin use after pancreatic cancer diagnosis is associated with enhanced survival (46,47). Our results are consistent with the reported protective role of statins that has been described. However, our data does not include the duration and dose of statin therapy or patient adherence to therapy. This limits our ability to interpret this association.
In our study, patient-level factors played a more significant role in mPCA survival, suggesting treatment approaches have a higher impact on survival compared to nSES measures and even well-known patient-level risk factors, including diabetes or obesity (48). This is evidenced by the fact that ROC curve estimates were lower when including nSES variables in the survival model. This suggests there could be a point along the cancer continuum where environmental effects or patient comorbidities may cease to impact cancer outcomes. The majority of studies conducted in nSES and cancer emphasize the impact of nSES on disease development, with less of an emphasis on progression or response to treatments (23,24,49-56). Thus, it’s possible that these factors may have less of a role in the setting of advanced cancer or that the short survival of our patient cohort limited the ability to evaluate the effect of these neighborhood variables. That is, under the chronic stress hypothesis, constant exposure to stressful environments could lead to cancer initiation and progression, but once the disease progresses to a certain stage, environmental effects are minimized (57,58). Additional studies would be needed to test this hypothesis in larger more heterogeneous cohorts.
While this study included detailed patient and nSES data, it had a number of limitations. The nSES variables selected in this study are commonly used in cancer studies (11), but there is no agreed upon, standard variable selection process in nSES and cancer research, which can limit the consistency and comparability across studies (59). We were unable to investigate all patient-level variables used in previous mPCA survival studies due to data availability (60,61). However, our study was the first to consider nSES together with patient-level factors and future studies that incorporate addition clinical and nSES variables are warranted. Additionally, the sample size was small and lacked representation by relevant subgroups, including race/ethnicity and stage. Further, this was a single institution study and therefore does not account for regional variations. The majority of patients who present to our center are medically insured, which limited our conclusions and investigations into the effects of health care access on PCA survival. All patients included in this study had metastatic disease while previous literature suggests that nSES effects could differ by stage of disease (62,63). Thus, conducting this analysis in a cohort with more representation from all disease stages, and across race/ethnic and SES gradients may be warranted.
While the results of this study do not fully characterize the relationship between social determinants of health and survival of patients with mPCA, the methodology used represents a novel and potentially clinically useful technique for identifying at risk patient populations. Social determinants of health, like the nSES data used in this study, are rarely, if ever, used by clinicians to guide treatment decisions for their patients. Utilizing information about a patient’s economic, physical and social environment can enhance patient care by taking a more holistic approach; one with a more complete understanding of the factors that influence a patient’ health. While nSES data is readily available through archives such as the U.S. Census, it’s accessibility to clinicians is limited by lack of a strategy to combine this trove of data with more familiar clinical variables. Geocoding can provide a link between clinical data and the social and physical environmental factors. Research that provides insight into the effect of social determinants of health, like neighborhood, on health outcomes would provide clinicians with another resource to help deliver personalized care to patients, particularly to those patients on a disparity-related pathway to disease, who may be at risk for poor outcomes due to socioeconomic circumstances.
Conclusions
In this study, there was suggestion of decreased survival in patients residing in more stable neighborhoods. However, the addition of nSES data to survival models with patient-level variables did not improve prediction accuracy. Consistent with prior data, our results demonstrate that age, receipt of chemotherapy, and initial surgical resection of the primary tumor were significant predictors of survival in this patient population, along with statin use. Further research is warranted to examine nSES effects on mPCA in a larger, more diverse cohort of patients.
Acknowledgments
Funding: This work was supported by generous funds from the Concetta and Chet Greenberg Institute of Pancreatic Cancer Research at Fox Chase Cancer Center; the Department of Defense Career Development Award (W81XWH-17-1-0276 to SML); and the National Cancer Institute Comprehensive Cancer Center Support Grant (CA06927 to Richard Fisher).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at: http://dx.doi.org/10.21037/jgo-20-39
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-20-39). The authors have no conflicts of interest to disclose.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Retrospective collection of patient data was approved by Fox Chase Cancer Center IRB (IRB #11-815). A waiver of informed consent was obtained from the IRB due to the study’s retrospective design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- SEER Cancer Stat Facts: Pancreatic Cancer. Bethesda, MD: National Cancer Institute. Accessed December 4, 2019.
- Khawja SN, Mohammed S, Silberfein EJ, et al. Pancreatic cancer disparities in African Americans. Pancreas 2015;44:522-7. [Crossref] [PubMed]
- Fesinmeyer MD, Austin MA, Li CI, et al. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2005;14:1766-73. [Crossref] [PubMed]
- Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg 2003;237:74-85. [Crossref] [PubMed]
- Zell JA, Rhee JM, Ziogas A, et al. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev 2007;16:546-52. [Crossref] [PubMed]
- Cheung MC, Yang R, Byrne MM, et al. Are patients of low socioeconomic status receiving suboptimal management for pancreatic adenocarcinoma? Cancer. 2010;116:723-33. [Crossref] [PubMed]
- Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci 2010;1186:125-45. [Crossref] [PubMed]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998;338:171-9. [Crossref] [PubMed]
- Byers TE, Wolf HJ, Bauer KR, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer 2008;113:582-91. [Crossref] [PubMed]
- Gomez SL, Hurley S, Canchola AJ, et al. Effects of marital status and economic resources on survival after cancer: A population-based study. Cancer 2016;122:1618-25. [Crossref] [PubMed]
- Gomez SL, Shariff-Marco S, DeRouen M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer 2015;121:2314-30. [Crossref] [PubMed]
- Shapiro M, Chen Q, Huang Q, et al. Associations of Socioeconomic Variables With Resection, Stage, and Survival in Patients With Early-Stage Pancreatic Cancer. JAMA Surg 2016;151:338-45. [Crossref] [PubMed]
- van Roest MH, van der Aa MA, van der Geest LG, et al. The Impact of Socioeconomic Status, Surgical Resection and Type of Hospital on Survival in Patients with Pancreatic Cancer. A Population-Based Study in The Netherlands. PLoS One 2016;11:e0166449. [Crossref] [PubMed]
- Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009;301:2553-62. [Crossref] [PubMed]
- Batabyal P, Vander Hoorn S, Christophi C, et al. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol 2014;21:2453-62. [Crossref] [PubMed]
- Walker EJ, Ko AH, Holly EA, et al. Statin use and risk of pancreatic cancer: results from a large, clinic-based case-control study. Cancer 2015;121:1287-94. [Crossref] [PubMed]
- Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol 2012;23:1880-8. [Crossref] [PubMed]
- Hamada T, Yuan C, Yurgelun MB, et al. Family history of cancer, Ashkenazi Jewish ancestry, and pancreatic cancer risk. Br J Cancer 2019;120:848-54. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med 2002;55:125-39. [Crossref] [PubMed]
- Keegan TH, Shariff-Marco S, Sangaramoorthy M, et al. Neighborhood influences on recreational physical activity and survival after breast cancer. Cancer Causes Control 2014;25:1295-308. [Crossref] [PubMed]
- DeRouen MC, Schupp CW, Koo J, et al. Impact of individual and neighborhood factors on disparities in prostate cancer survival. Cancer Epidemiol 2018;53:1-11. [Crossref] [PubMed]
- Freedman VA, Grafova IB, Rogowski J. Neighborhoods and chronic disease onset in later life. Am J Public Health 2011;101:79-86. [Crossref] [PubMed]
- Yost K, Perkins C, Cohen R, et al. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001;12:703-11. [Crossref] [PubMed]
- Krieger N, Singh N, Waterman PD. Metrics for monitoring cancer inequities: residential segregation, the Index of Concentration at the Extremes (ICE), and breast cancer estrogen receptor status (USA, 1992-2012). Cancer Causes Control 2016;27:1139-51. [Crossref] [PubMed]
- Census Tracts. Available online: https://www2.census.gov/geo/pdfs/education/CensusTracts.pdf
- Technical Documentation: Census 2000 Summary File 3. Technical Documentation/prepared by the U.S. Census Bureau, 2002.
- U.S. Census. Quick Facts. 2014. Available online: http://quickfacts.census.gov/qfd/meta/long_fips.htm
- Therneau T. Mixed Effects Cox Models. 2020. Available online: https://cran.r-project.org/web/packages/coxme/ vignettes/coxme.pdf
- Therneau T, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer-Verlag; 2000.
- Pyke DA, Thompson JN. Statistical-Analysis of Survival and Removal Rate Experiments. Ecology 1986;67:240-5. [Crossref]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29-36. [Crossref] [PubMed]
- Burnham KP, Anderson DR. Multimodel inference - understanding AIC and BIC in model selection. Sociol Method Res 2004;33:261-304. [Crossref]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Schulz AJ, Zenk SN, Israel BA, et al. Do neighborhood economic characteristics, racial composition, and residential stability predict perceptions of stress associated with the physical and social environment? Findings from a multilevel analysis in Detroit. J Urban Health 2008;85:642-61. [Crossref] [PubMed]
- Chaix B, Rosvall M, Merlo J. Neighborhood socioeconomic deprivation and residential instability: effects on incidence of ischemic heart disease and survival after myocardial infarction. Epidemiology 2007;18:104-11. [Crossref] [PubMed]
- Beck A, Davidson AJ, Xu S, et al. A Multilevel Analysis of Individual, Health System, and Neighborhood Factors Associated with Depression within a Large Metropolitan Area. J Urban Health 2017;94:780-90. [Crossref] [PubMed]
- Carrato A, Falcone A, Ducreux M, et al. A Systematic Review of the Burden of Pancreatic Cancer in Europe: Real-World Impact on Survival, Quality of Life and Costs. J Gastrointest Cancer 2015;46:201-11. [Crossref] [PubMed]
- Lambe M, Eloranta S, Wigertz A, et al. Pancreatic cancer; reporting and long-term survival in Sweden. Acta Oncol 2011;50:1220-7. [Crossref] [PubMed]
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846-61. [Crossref] [PubMed]
- Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg 1999;189:1-7. [Crossref] [PubMed]
- Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer 2005;5:930-42. [Crossref] [PubMed]
- Matusewicz L, Meissner J, Toporkiewicz M, et al. The effect of statins on cancer cells--review. Tumour Biol 2015;36:4889-904. [Crossref] [PubMed]
- Mullen PJ, Yu R, Longo J, et al. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer 2016;16:718-31. [Crossref] [PubMed]
- Zhang Y, Liang M, Sun C, et al. Statin Use and Risk of Pancreatic Cancer: An Updated Meta-analysis of 26 Studies. Pancreas 2019;48:142-50. [Crossref] [PubMed]
- Lee HS, Lee SH, Lee HJ, et al. Statin Use and Its Impact on Survival in Pancreatic Cancer Patients. Medicine 2016;95:e3607. [Crossref] [PubMed]
- Jeon CY, Pandol SJ, Wu B, et al. The association of statin use after cancer diagnosis with survival in pancreatic cancer patients: a SEER-medicare analysis. PLoS One 2015;10:e0121783. [Crossref] [PubMed]
- Arslan AA. Anthropometric Measures, Body Mass Index, and Pancreatic Cancer: A Pooled Analysis From the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010;170:791. [Crossref] [PubMed]
- Keegan TH, John EM, Fish KM, et al. Breast cancer incidence patterns among California Hispanic women: differences by nativity and residence in an enclave. Cancer Epidemiol Biomarkers Prev 2010;19:1208-18. [Crossref] [PubMed]
- Chang ET, Yang J, Alfaro-Velcamp T, et al. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev 2010;19:3106-18. [Crossref] [PubMed]
- Chang ET, Gomez SL, Fish K, et al. Gastric cancer incidence among Hispanics in California: patterns by time, nativity, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev 2012;21:709-19. [Crossref] [PubMed]
- Froment MA, Gomez SL, Roux A, et al. Impact of socioeconomic status and ethnic enclave on cervical cancer incidence among Hispanics and Asians in California. Gynecol Oncol 2014;133:409-15. [Crossref] [PubMed]
- Clarke CA, Glaser SL, Gomez SL, et al. Lymphoid malignancies in U.S. Asians: incidence rate differences by birthplace and acculturation. Cancer Epidemiol Biomarkers Prev 2011;20:1064-77. [Crossref] [PubMed]
- Horn-Ross PL, Lichtensztajn DY, Clarke CA, et al. Continued rapid increase in thyroid cancer incidence in California: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev 2014;23:1067-79. [Crossref] [PubMed]
- Ladabaum U, Clarke CA, Press DJ, et al. Colorectal cancer incidence in Asian populations in California: effect of nativity and neighborhood-level factors. Am J Gastroenterol 2014;109:579-88. [Crossref] [PubMed]
- Eschbach K, Mahnken JD, Goodwin JS. Neighborhood composition and incidence of cancer among Hispanics in the United States. Cancer 2005;103:1036-44. [Crossref] [PubMed]
- Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol 2010;6:1863-81. [Crossref] [PubMed]
- Reiche EM, Morimoto HK, Nunes SM. Stress and depression-induced immune dysfunction: implications for the development and progression of cancer. Int Rev Psychiatry 2005;17:515-27. [Crossref] [PubMed]
- Lynch SM, Mitra N, Ross MA, et al. A Neighborhood-Wide Association Study (NWAS): Example of Prostate Cancer Aggressiveness. PLoS One 2017;12:e0174548. [Crossref] [PubMed]
- Forssell H, Wester M, Åkesson K, et al. A proposed model for prediction of survival based on a follow-up study in unresectable pancreatic cancer. BMJ Open 2013;3:e004064. [Crossref] [PubMed]
- Hang J, Wu L, Zhu L, et al. Prediction of overall survival for metastatic pancreatic cancer: Development and validation of a prognostic nomogram with data from open clinical trial and real- world study. Cancer Med 2018;7:2974-84. [Crossref] [PubMed]
- Keegan THM, DeRouen MC, Parsons HM, et al. Impact of Treatment and Insurance on Socioeconomic Disparities in Survival after Adolescent and Young Adult Hodgkin Lymphoma: A Population-Based Study. Cancer Epidemiol Biomarkers Prev 2016;25:264-73. [Crossref] [PubMed]
- Tao L, Ladabaum U, Gomez SL, et al. Colorectal cancer mortality among Hispanics in California: Differences by neighborhood socioeconomic status and nativity: Colorectal Cancer Mortality in Hispanics. Cancer 2014;120:3510-8. [Crossref] [PubMed]


