Intrapancreatic accessory spleen: utilization of fine needle aspiration for diagnosis of a potential mimic of a pancreatic neoplasm
Introduction
Accessory spleen (AS) is a congenital anomaly in which there is failure of fusion between a portion of the developing splenic tissue and the main body of the spleen. ASs are present in approximately 10-15% of the population and are often an incidental finding (1,2). The majority of cases occur at the splenic hilum, while the second most common site is the tail of the pancreas (1,2). Although ASs are usually asymptomatic and clinically innocuous, their presence may be noted incidentally on radiologic imaging. When located within the tail of the pancreas, they may mimic a pancreatic neoplasm such as a neuroendocrine tumor, solid pseudopapillary tumor or pancreatic adenocarcinoma (3). For this reason, it is important to be able to differentiate accessory splenic tissue from other pancreatic neoplasms to avoid unnecessary surgery.
Radiologically, intrapancreatic ASs (IPAS) tend to be well-defined, small (1-3 cm), hypervascular lesions with imaging characteristics similar to that of normal spleen. Importantly, on imaging, IPAS usually remain stable over time. Although imaging modalities continue to improve throughout the years, it may not always be possible to definitely diagnose IPAS by imaging, necessitating a biopsy for further classification of the lesion (4).
With the ability to use endoscopic ultrasound to guide biopsies, fine needle aspiration (FNA) has become very important in the diagnosis of pancreatic lesions. Specifically, in regards to IPAS, more recently there have been case reports describing its diagnosis via FNA within the cytopathology literature. A recent review of documented IPAS cases diagnosed by FNA showed the common cytologic features to include a heterogenous population of lymphocytes (often with background eosinophils, histiocytes and plasma cells) along with traversing small vessels (5). Perhaps one of the most helpful features that has been described in the diagnosis of IPAS is the use of CD8 immunohistochemical staining. The endothelial cells of the splenic sinus, which are often well-demonstrated on cell block material, stain positive for CD8, a commonly used T-cell marker (6,7). It has been reported that this staining is specific since systemic endothelial cells and hemangiomas are negative and can therefore be a helpful confirmatory test (8).
Case presentation
Case 1
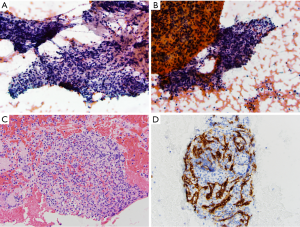
A 32-year-old woman presented with increasing abdominal pain. Work-up included an MRI which demonstrated a 1.4 cm × 2.0 cm T2 hyperintense enhancing lesion in the tail of the pancreas, concerning for a neuroendocrine tumor. Endoscopic ultrasound at the time of FNA biopsy revealed a 2 cm oval, well-defined, hypoechoic, homogenous mass in the tail of the pancreas. Alcohol-fixed smears, along with cell block material, showed a population of small-to-medium lymphocytes, consistent with chronic inflammation. Given that these findings were not concordant with the presence of a mass, a repeat FNA biopsy was performed. Alcohol-fixed smears and cell block material from this second sample also showed a polymorphic population of lymphocytes along with eosinophils, neutrophils and histiocytes (Figure 1A-C). In order to differentiate between a lymph node and IPAS, an immunohistochemical stain for CD8 was performed on the cell block material, which highlighted splenic sinus endothelial cells, supporting the diagnosis of IPAS (Figure 1D).
Case 2
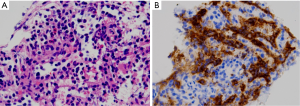
A 57-year-old woman was found to have an incidental solid pancreatic tail mass and underwent an endoscopic ultrasound-guided FNA. Aspirate smears and cell block material were sent to UCSF for consultation in order to rule out a mucinous neoplasm. Aspirate smears contained mucoid material and small fragments of columnar epithelium. Cell block material demonstrated predominantly lymphocytic elements (Figure 2A), as confirmed by CD45 stain. Immunohistochemical stains for neuroendocrine markers were negative. Given the presence of the mucinous epithelium, the possibility of a mucinous neoplasm was raised. However, given the solid nature of the lesion, this was thought to be less likely and was more likely to represent contaminating gastric mucosa secondary to entering the pancreas from the stomach during the procedure. A CD8 stain performed on the cell block material revealed positive staining in sinusoidal endothelial cells, confirming the diagnosis of IPAS (Figure 2B).
Discussion
The presence of an AS is not a rare occurrence, and with the second most common site being the tail of the pancreas, it is important to be able to recognize IPAS for proper diagnosis and subsequent management. With the increased use of endoscopic ultrasound-guided FNA for diagnosis of pancreatic masses, the cytologic features of IPAS have been recently reported in the literature. Above, we report two additional cases diagnosed via FNA. Both cases demonstrated the following common features of IPAS: a heterogenous population of small lymphocytes and other hematopoietic cells with a background of traversing small vessels that stain with CD8, confirming sinusoidal endothelial cells. Recognition of these features, along with correlation with radiologic studies, allows for a diagnosis of IPAS with avoidance of unnecessary surgical resection.
Although many of the primary pancreatic neoplasms enter into the differential diagnosis of IPAS, pancreatic neuroendocrine tumors are at the top of the list. A recent case report describes the presence of fine granular (salt-and-pepper) chromatin and poorly formed pseudorosette structures within an IPAS, the findings of which were suspicious for a neuroendocrine tumor. However, a subsequent distal pancreatectomy revealed IPAS, highlighting the overlap that may exist (5). Using the common cytomorphologic findings described above, in combination with immunohistochemical staining for CD8 and the neuroendocrine markers synaptophysin and chromogranin will improve diagnostic accuracy. A word of caution should be advised when using chromogranin and synaptophysin positivity to support a diagnosis of neuroendocrine tumor, as the presence of background benign pancreatic islet cells will also stain positively. Therefore, as with all immunohistochemical stains, correlation with the morphologic findings is essential.
As highlighted in case 2 above, contaminating gastric epithelium may also raise the possibility of a mucinous neoplasm. Lesions within the pancreatic tail require endoscopic ultrasound sampling through the stomach which may result in the introduction of fragments of gastric mucosa into the FNA material. Correlation with imaging characteristics to determine whether the lesion is solid or cystic can be of utmost importance.
As an interesting side note, while ASs are a result of a congenital anomaly and may result in an intrapancreatic mass, it is also important to recognize the potential for auto-transplantation of splenic tissue as a result of trauma or iatrogenic rupture of the spleen. This may lead to deposition of splenic tissue throughout the abdomen, resulting in single or multiple nodules mimicking malignancies such as lymphoma and metastatic carcinoma (9). Similar to IPAS, case reports of intrahepatic splenosis mimicking hepatocellular carcinoma have been reported (10), including a case diagnosed by FNA (11). The authors describe similar cytologic findings to those of IPAS with a mixture of hematopoietic cells (including lymphocytes, neutrophils, eosinophils, histiocytes and plasma cells) with a background of vascular-like structures (11).
In conclusion, IPAS is a benign congenital anomaly that may mimic a pancreatic neoplasm. FNA can be performed for diagnosis. The use of a combination of common cytomorphologic features, immunohistochemical stains and radiologic findings will enable an accurate diagnosis and prevent unnecessary surgical procedures.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Paul M. Accessory spleens. Lancet 1937;2:74-77.
- Halpert B, Alden ZA. Accessory spleens in or at the tail of the pancreas. A survey of 2,700 additional necropsies. Arch Pathol 1964;77:652-4. [PubMed]
- Arkadopoulos N, Athanasopoulos P, Stafyla V, et al. Intrapancreatic accessory spleen issues: diagnostic and therapeutic challenges. JOP 2009;10:400-5. [PubMed]
- Spencer LA, Spizarny DL, Williams TR. Imaging features of intrapancreatic accessory spleen. Br J Radiol 2010;83:668-73. [PubMed]
- Rodriguez E, Netto G, Li QK. Intrapancreatic accessory spleen: a case report and review of literature. Diagn Cytopathol 2013;41:466-9. [PubMed]
- Lin J, Jing X. Fine-needle aspiration of intrapancreatic accessory spleen, mimic of pancreatic neoplasms. Arch Pathol Lab Med 2010;134:1474-8. [PubMed]
- Schreiner AM, Mansoor A, Faigel DO, et al. Intrapancreatic accessory spleen: mimic of pancreatic endocrine tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy. Diagn Cytopathol 2008;36:262-5. [PubMed]
- Kraus MD. Splenic histology and histopathology: an update. Semin Diagn Pathol 2003;20:84-93. [PubMed]
- Fremont RD, Rice TW. Splenosis: a review. South Med J 2007;100:589-93. [PubMed]
- Yu H, Xia L, Li T, et al. Intrahepatic splenosis mimicking hepatoma. BMJ Case Rep 2009;2009.
- Galloro P, Marsilia GM, Nappi O. Hepatic splenosis diagnosed by fine-needle cytology. Pathologica 2003;95:57-9. [PubMed]


