Non-pancreatic retroperitoneal mucinous neoplasms and a discussion of the differential diagnosis
Introduction
Retroperitoneal masses are a diverse group of lesions that range from benign to malignant. These lesions can be solid or cystic, single or multiloculated, and the contents may range from serous to mucinous fluid. Some nonneoplastic lesions include pancreatic pseudocyst, non-pancreatic pseudocyst, lymphocele, urinoma, and hematoma, while neoplastic lesions include cystic lymphangioma, mucinous cystadenoma, cystic teratoma, cystic mesothelioma, Mullerian cyst, epidermoid cyst, bronchogenic cyst, cystic change in solid neoplasms, pseudomyxoma retroperitonei and perianal mucinous carcinoma (1), as well as primary pancreatic tumors, including mucinous types. The clinical implications and treatment options vary depending on the diagnosis, and therefore, differentiating these lesions is imperative. Clinical and surgical history along with radiographic results should be reviewed and considered.
Case findings
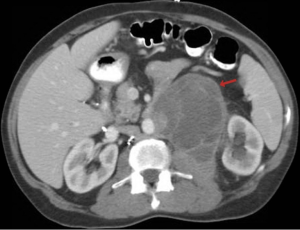
One year ago a 51-year-old man underwent a cholecystectomy at an outside facility for symptomatic cholelithiasis and cholecystitis. During the surgical procedure a biopsy was performed on a suspected pancreatic pseudocyst and was diagnosed as a low-grade mucinous tumor of probable pancreatic origin. The patient subsequently received chemotherapy for “locally advanced pancreatic cancer”. One year later he presented to our institution and underwent retroperitoneal en bloc resection of the residual mass. Radiologically, and at the time of surgery the neoplasm did not involve the pancreas (Figure 1). At our institution’s multidisciplinary tumor board additional medical history was obtained, and review of the patient’s chart then revealed a remote history of left orchiectomy for a testicular tumor excised 25 years prior.
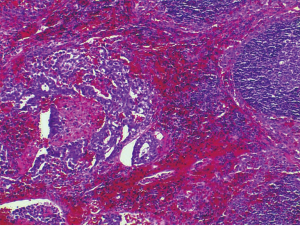
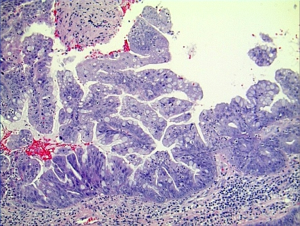
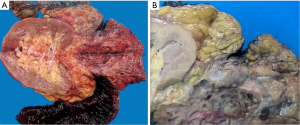
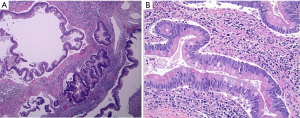
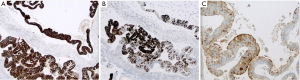
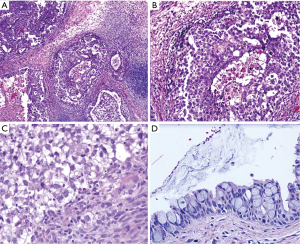
Histologically the initial abdominal mass biopsy from the outside facility showed glands ranging from complex cytologically malignant proliferations to those lined by a single layer of bland epithelium with predominately mucinous features (Figure 2). Gross findings of the subsequent retroperitoneal en bloc resection performed at our institution included a 7 cm solid and multiloculated cystic tumor adherent to the small and large intestines, wall of the aorta and para-aortic tissues (Figure 3). The mass was 0.5 cm away from the inferior pole of the left kidney. Notably the resection did not contain any identifiable pancreatic tissue. Microscopically the retroperitoneal resection showed similar features to the initial case, differing primarily in that it had a relatively lower percentage of the more atypical epithelium, likely due to treatment effect with chemotherapy (Figure 4). Immunohistochemistry showed non-uniform positivity in the glands for cytokeratin 7 (CK7), cytokeratin 20 (CK20) and cancer antigen 19.9 (CA19.9) (Figure 5). Review of the archival slides from the testicular tumor and subsequent para-aortic lymph node dissection showed a mixed germ cell tumor with embryonal, yolk sac, seminomatous and teratomatous components with focal bland mucinous epithelium (Figure 6); microscopic foci of metastatic embryonal carcinoma were identified in 2 of 34 retroperitoneal para-aortic lymph nodes (Figure 7).
Differential diagnosis
As previously mentioned, there is a wide differential diagnosis for retroperitoneal lesions. Nonneoplastic lesions include pancreatic pseudocyst, nonpancreatic pseudocyst, lymphocele, urinoma and hematoma. Clinically pancreatic pseudocysts commonly occur in patients that have had a history of recurrent pancreatitis, and concurrent laboratory studies will often show high levels of amylase or lipase; radiographically a lesion is seen in the peripancreatic space. In contrast nonpancreatic pseudocysts are asymptomatic clinically, but show a lesion with a thick fibrous wall on computed tomography (CT). The history that may be associated with lymphocele is a patient who is status post radical lymphadenectomy, whereas both urinomas and hematomas are associated with trauma.
The neoplastic differential includes, but is not limited to cystic lymphangioma, mucinous cystadenoma, cystic teratoma, cystic mesothelioma, Mullerian cyst, epidermoid cyst, bronchogenic cyst, cystic change in solid neoplasms, and pseudomyxoma retroperitonei. Mucinous cystadenoma is typically asymptomatic, found predominately in women and unilocular radiographically. However, there have been case reports of mucinous cystadenomas occurring in male patients as well as rare incidences of malignant transformation (2-4). Cystic teratoma and epidermoid cyst are also asymptomatic and more common in females. Cystic teratomas often show the presence of fat and calcifications on CT. Cystic change in solid neoplasms is an important consideration in patients with a known malignancy or in patients that have a large tumor burden with focal cystic changes. Patients with pseudomyxoma retroperitonei will present with a palpable mass and abdominal pain; radiographically there will be a multicystic lesion with thick septations and curvilinear calcifications.
Discussion
This case illustrates the significance of obtaining a full clinical and surgical history, however remote, as well as being able to easily acquire medical records and archived cases. This case also demonstrates the importance of working closely with other specialty departments, which in this case noted that the neoplasm did not involve the pancreas radiologically or at the time of surgery. The retroperitoneal mucinous neoplasm showed positive staining for CK7, CK20 and CA19.9, which is consistent with a primary pancreatic tumor. However, a similar immunohistochemical profile may also be seen with non-pancreatic mucinous neoplasms, including tumors arising from testicular teratomatous tissues, ovarian mucinous tumors and primary retroperitoneal mucinous cystic neoplasms (2,5).
Most primary retroperitoneal mucinous tumors are malignant (4). Rare, yet significant differentials of this case include primary retroperitoneal cystadenoma, mucinous cystadenoma of borderline malignancy and cystadenocarcinoma. If there had been no history of a malignant testicular germ cell tumor with retroperitoneal lymph node metastases, this case would have been classified as a probable primary retroperitoneal mucinous cystic neoplasm. Microscopically, these neoplasms show dilated spaces lined by mucinous epithelium ranging from bland to frankly malignant and case reports demonstrate positive immunohistochemistry with both CK7 and CK20 (6). However, in a reported case of a child, where the lesion was thought to be Mullerian in origin, immunohistochemical stains were positive for CK7 and CA19.9 but negative for CK20 (7).
There are four general theories regarding the pathogenesis of primary retroperitoneal cystadenomas. However, there is no unanimous opinion regarding the genesis of these tumors, therefore due to their rarity, the histogenesis, biologic behavior and optimal management strategies remain at a speculative level (6). One theory suggests the seeding of ectopic ovarian tissue in the retroperitoneum (8). However, ovarian tissue has rarely been identified in these lesions, and similar cases have now been reported in several male patients (2-4). A second hypothesis suggests that these tumors result from invagination of the peritoneal lining with entrapment of multipotent mesothelial cells that subsequently undergo mucinous metaplasia and cyst formation (9-11). Thirdly, some believe the neoplasm may originate from an enterogenic duplication cyst (6). The final hypothesis is that these retroperitoneal cystic lesions develop as a result of a teratoma, in which the mucinous epithelium has overgrown all other tissue types (3).
Growing teratoma syndrome describes the phenomenon in which an individual with a history of a non-seminomatous germ cell tumor has an enlarging metastatic mass which is resistant to chemotherapy and has normalized serum markers (12). These are most commonly observed in the retroperitoneum, but have also been described in the mediastinum, supraclavicular lymph nodes, mesentery and other locations including visceral organs (12). The presence of teratomatous components in the original orchiectomy specimen is reported to be as high as 86% in growing teratoma syndrome (13). Malignant transformation within a teratoma is an unusual occurrence that is seen in less than one percent of cases, most often seen with the more commonly occurring ovarian teratomas developing squamous cell carcinoma. However, in men with retroperitoneal teratomas 3% to 6% undergo transformation into more malignant neoplasms such as sarcoma, adenocarcinoma or primitive neuroectodermal tumors (14,15). Chemotherapy is often one of the treatment modalities for metastatic testicular germ cell tumors. Patients may have an incomplete response to chemotherapy, in that the slow growing teratomatous component may be more resistant to therapy than the more proliferative malignant germ cell elements and thus persist after treatment and potentially become a site of secondary malignancy or recurrence. Secondary malignancies which arise in a teratoma are generally resistant to chemotherapy, therefore complete surgical resection is the recommended treatment (16,17).
A thorough history correlated with imaging is imperative to the development of differential diagnoses. The mucinous glandular component of the primary testicular tumor in this case was very minor, with primarily small glands without definite differentiation along an organ type. Additional tissue from the current en bloc resection was submitted to attempt to identify germ cell and nonmucinous components, which would better support a carcinoma arising within a proliferating/growing teratoma, but only mucinous and simple glandular tissues were present. No ovarian type stroma was identified. With no clinical evidence of tumor in the pancreas or extension from it, the predominantly para-aortic location of the tumor favors a carcinoma arising from a rest of mature metastatic teratoma that persisted after chemotherapy many years ago. This case illustrates the importance of a thorough history correlated with imaging in the development of differential diagnoses and the need to consider primary as well as metastatic nonpancreaticobiliary sources of retroperitoneal mucinous tumors (18).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yang DM, Jung DH, Kim H, et al. Retroperitoneal cystic masses: CT, clinical, and pathologic findings and literature review. Radiographics 2004;24:1353-65. [PubMed]
- Thamboo TP, Sim R, Tan SY, et al. Primary retroperitoneal mucinous cystadenocarcinoma in a male patient. J Clin Pathol 2006;59:655-7. [PubMed]
- Benkirane A, Mikou A, Jahid A, et al. Primary retroperitoneal mucinous cystadenoma with borderline malignancy in a male patient: a case report. Cases J 2009;2:9098. [PubMed]
- Falidas E, Konstandoudakis S, Vlachos K, et al. Primary retroperitoneal mucinous cystadenoma of borderline malignancy in a male patient. Case report and review of the literature. World J Surg Oncol 2011;9:98. [PubMed]
- Alasio TM, Borin J, Taylor K, et al. Intratesticular mucinous cystadenoma: immunohistochemical comparison with ovarian and colonic tissue. Arch Pathol Lab Med 2005;129:399-402. [PubMed]
- Bifulco G, Mandato VD, Giampaolino P, et al. Huge primary retroperitoneal mucinous cystadenoma of borderline malignancy mimicking an ovarian mass: case report and review. Anticancer Res 2008;28:2309-15. [PubMed]
- Behr C, Hesketh A, Soffer S, et al. Primary retroperitoneal mucinous cystadenoma: An unusual cause of an abdominal mass in a child. J Pediatr Surg Case Rep 2014;2:61-3.
- Matsubara M, Shiozawa T, Tachibana R, et al. Primary retroperitoneal mucinous cystadenoma of borderline malignancy: a case report and review of the literature. Int J Gynecol Pathol 2005;24:218-23. [PubMed]
- Jiang H, Jin K, You Q, et al. Retroperitoneal primary mucinous adenocarcinoma: A case report. Oncol Lett 2011;2:633-6. [PubMed]
- Subramony C, Habibpour S, Hashimoto LA. Retroperitoneal mucinous cystadenoma. Arch Pathol Lab Med 2001;125:691-4. [PubMed]
- Navin P, Meshkat B, McHugh S, et al. Primary retroperitoneal mucinous cystadenoma-A case study and review of the literature. Int J Surg Case Rep 2012;3:486-8. [PubMed]
- Gorbatiy V, Spiess PE, Pisters LL. The growing teratoma syndrome: Current review of the literature. Indian J Urol 2009;25:186-9. [PubMed]
- Tangjitgamol S, Manusirivithaya S, Leelahakorn S, et al. The growing teratoma syndrome: a case report and a review of the literature. Int J Gynecol Cancer 2006;16:384-90. [PubMed]
- Lavery HJ, Bahnson RR, Sharp DS, et al. Management of the residual post-chemotherapy retroperitoneal mass in germ cell tumors. Ther Adv Urol 2009;1:199-207. [PubMed]
- Kim JH, Lee TS, Oh HK, et al. A case of mucinous adenocarcinoma arising from retroperitoneal teratoma treated with chemoradiation. J Gynecol Oncol 2009;20:126-8. [PubMed]
- Carver BS, Shayegan B, Serio A, et al. Long-term clinical outcome after postchemotherapy retroperitoneal lymph node dissection in men with residual teratoma. J Clin Oncol 2007;25:1033-7. [PubMed]
- Damjanov I, Hes O. The Effects of Chemotherapy on Metastatic Testicular Germ Cell Tumors. The Open Pathology Journal 2009;3:45-52.
- Blount SE, Conrad R, Cobb C, et al. Late Mucinous Tumor Arising in a Retroperitoneal Proliferating Teratoma in a Man, Clinically and Immunohistochemically Mimicking a Pancreatic Lesion: A Diagnostic Dilemma (Poster No. 125). Abstracts and Case Studies from the College of American Pathologists 2013 Annual Meeting. Arch Pathol Lab Med 2013;137:1425.