|
Case Report
Unusual metastases of gastrointestinal stromal tumor and genotypic correlates: Case report and review of the literature
Sonia M Abuzakhm, Carlos E Acre-Lara, Weiqiang Zhao, Charles Hitchcock, Nehad Mohamed, Daria Arbogast, Manisha H Shah
Department of Internal Medicine and Department of Pathology, The Ohio State University, Columbus, OH
Corresponding to: Sonia M Abuzakhm, MD. 406C: Office 1 Starling Loving Hall, 320 West 10th Avenue, Columbus, OH 43210. Tel: 614-293-8858; Fax: 614-293-7484. Email: sonia.abuzakhm@osumc.edu.
J Gastrointest Oncol 2011; 2: 45-49. DOI: 10.3978/j.issn.2078-6891.2011.006
|
|
Introduction
Gastrointestinal stromal tumors (GISTs) are defined as
mesenchymal tumors of the gastrointestinal tract and are
characterized by positive CD117 staining, and in most cases
positive CD34 staining, with compatible gross features
and microscopic findings of a highly cellular mesenchymal
tumor of the gastrointestinal tract composed of spindle
cells, epithelioid cells or a combination of both ( 1). They
are usually derived from a mutation of the KIT (CD117) or
PDGFRA (platelet derived growth factor receptor alpha)
gene. Distinguishing GIST from other mesenchymal
derived tumors was historically a challenge, since both can
arise from the interstitial cells of Cajal, or GI pacemaker
cells that form the interface between the autonomic
innervation and smooth muscle of the bowel wall ( 2).
The distinction of GISTs based on molecular etiology
was described by Hirota et al in 1998, with discovery of
a mutation in c-KIT encoding a pro-oncogenic receptor
tyrosine kinase (KIT) ( 3). It is estimated that 4500 to 6000 new cases of GIST are
diagnosed in the United States annually and most occur in
the stomach (50%-70%) or small intestine (20%-30%) ( 4).
GISTs are often asymptomatic and discovered incidentally during surgery, endoscopic procedures, or imaging studies.
However, the clinical presentation of some GISTs may
include overt GI bleeding, abdominal mass, abdominal
pain, or bowel obstruction and acute abdomen ( 2). The
most common metastatic sites of gastrointestinal stromal
tumors are the liver (65%) and peritoneum (21%); GISTs
rarely metastasize to lymph nodes (6%), bone (6%), lung
(2%) ( 2, 5), and soft tissue (less than 1%) ( 6, 7). We report
the case of a female diagnosed with GIST with subsequent
metastases to the liver, peritoneum, lung, bone, and soft
tissue.
|
|
Case presentation
A 57 year-old Caucasian female , with history of
hypertension and diabetes mellitus, presented to an
emergency department (ED) in March 2003, with
complaints of acute onset of abdominal pain and three
month history of fatigue. Her evaluation revealed anemia
with hemoglobin of 6.8 gm/dL, and a small bowel
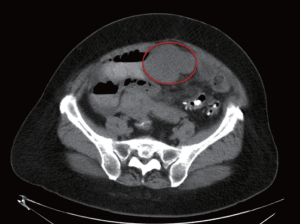
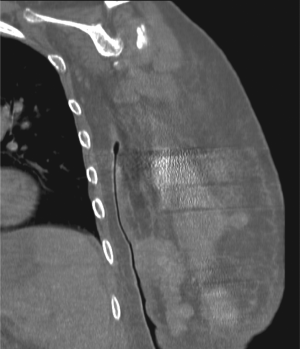
obstruction by CT imaging of the abdomen/pelvis (Fig 1).
She underwent a small bowel mass resection. Pathology
confirmed a gastrointestinal stromal tumor with a 9 cm
primary tumor in the jejunum. Immunohistochemistry
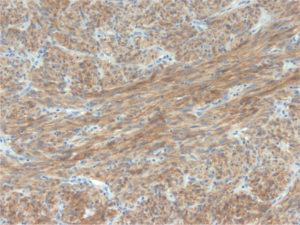
revealed spindle cells positive for CD117 (Fig 2) and CD34,
negative for S-100 protein, cytokeratin, and smooth muscle
myosin. Mitotic activity was low (
The patient was clinically stable and followed by serial
imaging until May 2004, when she complained of right
upper quadrant abdominal pain and a CT scan of the
abdomen revealed liver metastases. The patient began
treatment with oral imatinib mesylate (Gleevac) at a dose
of 400 mg/day, and a partial response was achieved for two years. The patient then experienced recurrence of right upper
quadrant pain and a CT scan demonstrated increase in the
size of liver metastases and a new pleural effusion. Subsequent
treatment was initiated with oral sunitinib malate at a dose of
50 mg/day, on a schedule of 28 days on and 14 days off. The
patient experienced significant side effects including fatigue,
severe mouth soreness, decreased appetite, and hand-foot
syndrome, necessitating dose reduction to oral sunitinib
malate at a dose of 37.5 mg/day after three cycles on the
initial dosage. Stable disease was achieved for approximately
twelve months while on oral sunitinib.
In April 2007, she had progression of disease in the
form of a pathological fracture of the lef t humerus.
Biopsy of the left humerus revealed a spindle cell sarcoma
morphologically consistent with GIST metastasis, however
immunohistochemical stains were negative for CD117
(c-KIT), CD34, and bcl-2. Sunitinib was discontinued preoperatively,
and the patient underwent reconstruction of the
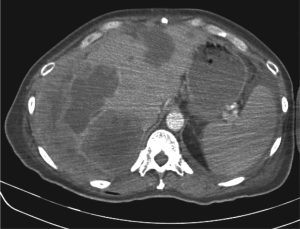
left distal humerus. A CT of the abdomen and pelvis in May
2007 showed dramatic progression of liver metastases (Fig
3). Given the progression of disease while being off sunitinib
and in the absence of other standard of care treatment, she
was restarted on oral sunitinib malate at a dose of 37.5 mg/
day, on a schedule of 28 days on and 14 days off. In August
2007, she developed hard nodules in the subcutaneous
area of the left upper extremity, concerning for tumor
recurrence. CT scan of the left humerus revealed multiple
soft tissue nodules scattered throughout the humerus (Fig
4). She continued sunitinib as systemic therapy and began
local radiation therapy of the left humerus for palliation.
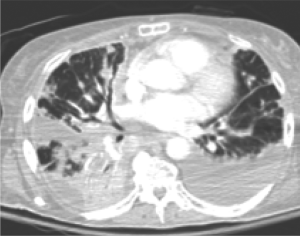
In October 2007, the patient was hospitalized for dyspnea, ascites, and lower extremity edema. Imaging
showed further metastases to the peritoneum and lungs and
bilateral pleural effusions (Fig 5). Despite two thoracenteses
and pleurodesis, she had progressive symptoms and
worsening lung nodules. Her respiratory failure was rapidly
progressive and she died in October 2007, approximately 55
months after her initial diagnosis.
Due to unusual sites of metastases, a limited autopsy
of the liver, lung and left arm tissue was performed after
written consent from her power of attorney. The lung and
liver metastatic lesions were morphologically consistent
with GIST, and immunohistochemica l sta ins were
positive for CD117 (c-KIT). Tumor cells from the left arm
subcutaneous nodule were morphologically suggestive of
GIST but negative for CD117 by immunohistochemical
staining. Molecular analysis demonstrated an in-frame
deletion of 74450-74455 (6bp), or del559V-560V (or
codons 559/560) in exon 11 of the KIT gene in sequences
from metastases of the right lung, left lung, liver, and left
arm subcutaneous nodule. No mutation in exon 18 of the
PDGFRA gene was identified in these metastases.
|
|
Review of the literature
Outside of a retrospective analysis conducted by Schuler
et al ( 5), which reported that seventeen out of 307 patients
with GIST had bone metastases, there are only a few
reported cases in the literature of patients with GIST
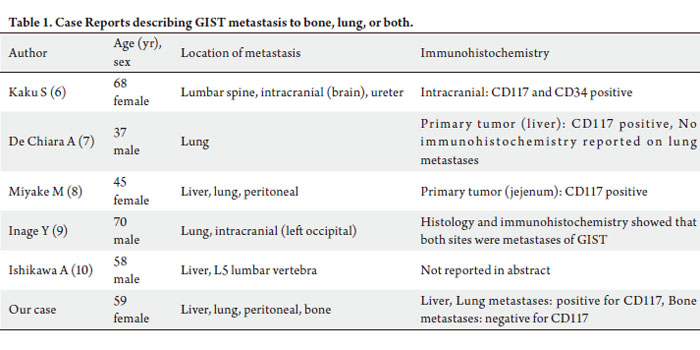
metastases to the bone, lung, or both (Table 1). Kaku
et al ( 8) described a case of a 68 year-old woman with
intracranial metastasis occurring two years after surgical resection of a GIST tumor of the sacrum. She subsequently
developed metastatic tumor involving the lumbar spine
and ureter. The intracranial metastasis was resected
by right parietal craniotomy and was c-KIT positive
by immunohistochemistry. Biopsy or surgery was not
performed on the lumbar spine and ureter lesions. A 37 yearold
man with primary GIST of the liver metastatic to the
lung is described by DeChiara et al ( 9). The primary tumor
was initially diagnosed as a high grade sarcoma, but after
further immunohistochemical study, the liver tumor cells
stained positively for c-KIT and the tumor was diagnosed as GIST. Fourteen months after this diagnosis, the patient was
found to have lung metastases by CT scan, and confirmed
by PET. While pathology and immunohistochemistry were
not reported on the lung metastases, it was reported that
the pulmonary lesions disappeared completely with oral
imatinib treatment, suggesting a similar molecular basis
of these lesions. Miyake et al ( 10), and Inage et al ( 11),
described patients with multiple sites of metastases, with
both patients having lung metastases. Ishikawa et al ( 12)
reported a patient with liver and bone metastases, in the
form of a lumbar vertebral lesion. With the exception of our
report, mutational studies of KIT and PDGFRA genes were
not reported in these five other cases ( 8-12). Even more rare than metastases to bone and lung,
metastases of GIST to subcutaneous tissue are reported
in less than 1% of cases ( 6, 7). In a series of patients with
stomach GIST, five out of 1765 patients (0.04%) developed
sk in or sof t tissue metastases ( 6). No patients were
reported to have soft tissue or skin metastases in a series
of 906 patients with small intestine GIST ( 7). Prior to
our reported case, the literature includes six case reports
( 13-18) describing ten patients with cutaneous metastases
as a late complication of GIST. The first reported case
( 13) described a 49 year-old male with multiple skin and subcutaneous metastases to the scalp, anterior jaw, left
thigh, and groin, along with liver and splenic metastases.
This report did not include description of microscopic,
immunohistochemical and molecular features. The
patient was treated with gemcitabine and thalidomide,
experienced a minimal response and was then lost to
follow up. Anagnostoulis et al ( 14) reported a 69 yearold
female who presented with synchronous gastric GIST
and a subcutaneous paraumbilical metastasis, proven by
histology and immunohistochemistry to be consistent with
GIST. She died four days postoperatively after gastrectomy
and resection of subcutaneous metastasis. Other reports
described three patients with subcutaneous metastases in
the parietal bone region ( 15), gluteal region (biopsy proven
and immunohistochemistry positive for CD117) ( 16), and
right upper arm (biopsy proven, immunohistochemistry
positive for CD117) ( 17) respectively. Outside of our article, the only other literature to
report subcutaneous metastasis of GIST and provide both
immunohistochemical and mutational analysis of the
subcutaneous metastases is a case series by Wang et al ( 18).
They describe two patients with abdominal cutaneous
metastases and three extra-abdominal cutaneous metastases
(two to scalp and one to cheek). All five cases had multiple
concurrent or subsequent abdominal and/or hepatic
metastases. Immunohistochemical studies for CD117
expression were performed on the cutaneous metastases
in all five cases, and all cases were positive for CD117. In
addition to this, four out of the five cases were analyzed for
KIT mutations in exons 9, 11, 13, and 17. Two of the four
cases had mutations in exon 11, and the remaining two cases were wild-type for exons 9, 11, 13, and 17.
|
|
Discussion
The development of molecularly targeted therapy against
c-KIT and PDGFRA with imatinib and sunitinib has
significantly altered the treatment of GIST. Notably,
imatinib has been shown to increase progression free
survival in advanced disease ( 19). Most of the somatic
mutations in c-KIT are gain-of-function mutations found
in exon 11 and exon 9, with exon 11 mutations showing
improved objective responses, time to tumor progression,
and overall survival in patients treated with imatinib ( 19). A
mutation in exon 11 was present in our patient’s malignancy,
and she experienced a time to tumor progression of
approx imately two yea rs whi le on imat inib. Wit h
progression to liver metastases, indicating imatinib resistant
GIST, she was started on sunitinib. Despite use of sunitinib,
her disease progressed in the form of lung and bone
metastases. The clinical activity of sunitinib after imatinib
failure has also been correlated with kinase genotype, with
progression-free survival and overall survival significantly
longer for patients with primary KIT exon 9 mutations or
with wild-type genotype, as compared to those with KIT
exon 11 mutations ( 20). While the relationship between certain kinase genotypes
and clinical progression has been described in articles by
Heinrich et al ( 19, 20), it remains unclear why some patients
develop particularly aggressive and unusual metastases. It is
also unclear why expression of CD117 in certain metastatic
lesions is diminished or absent, such as in our patient’s left arm subcutaneous nodule. The absence of CD117 may be
related to dedifferentiation of the malignancy or associated
with changes induced by tyrosine kinase inhibitor therapy.
Loss of CD117 expression has been observed in advanced
GIST cases, and may itself be a harbinger of imatinib failure
and poor prognosis ( 21, 22). We further postulate that the
type of mutation, including point, substitution, deletion, or
deletion-insertion, may affect clinical aggressiveness and
prognosis, as well as response to imatinib and sunitinib,
with exon 11 deletions having a more aggressive course.
Additional research is needed to elucidate the relationship
between the type of mutant genotypes, and the site of
metastases, clinical aggressiveness, and response to tyrosine
kinase inhibitors.
|
|
References
- Graadt van Roggen JF, van Velthuysen ML, Hogendoorn PC. The
histopathological differential diagnosis of gastrointestinal stromal
tumours. J Clin Pathol 2001;54:96-102.[LinkOut]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on
morphology, molecular pathology, prognosis, and differential diagnosis.
Arch Pathol Lab Med 2006;130:1466-78.[LinkOut]
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S,
et al. Gain-of-function mutations of c-KIT in human gastrointestinal
stromal tumors. Science 1998;279:577-80.[LinkOut]
- American Cancer Society [Internet]. Detailed Guide: Gastrointestinal
stromal tumor (GIST): What are the key statistics about
gastrointestinal stromal tumors; c2010 [updated 2010 August 24; cited
2010 October 15]. Available from: http://www.cancer.org/acs/groups/
cid/documents/webcontent/003103-pdf.pdf[LinkOut]
- Schuler M, Zeile M, Pink D, Tunn A, Kretzschmar A, Rau B, et
al. Incidence of bone metastases in GIST: A single center analysis
of 307 patients with metastatic disease [abstract]. J Clin Oncol
2008;s26:10565.
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of
the stomach: a clinicopathologic, immunohistochemical, and molecular
genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol
2005;29:52-68.[LinkOut]
- Miettinen M, Mak louf H, Sobin LH, Lasota J. Gastrointestinal
stromal tumors of the jejenum and ileum: a clinicopathologic,
immunohistochemical, and molecular genetic study of 906 cases before
imatinib with long-term follow-up. Am J Surg Pathol 2006;30:477-89.[LinkOut]
- Kaku S, Tanaka T, Ohtuka T, Seki K, Sawauchi S, Numoto RT, et al.
Perisacral gastrointestinal stromal tumor with intracranial metastasis:
case report. Neurol Med Chir (Tokyo) 2006;46:254-7.[LinkOut]
- De Chiara A, De Rosa V, Lastoria S, Franco R, Botti G, Iaffaioli VR,
et al. Primary gastrointestinal stromal tumor of the liver with lung
metastases successfully treated with STI-571 (imatinib mesylate). Front
Biosci 2006;11:498-501.[LinkOut]
- Miyake M, Takeda Y, Hasuike Y, Kashiwazaki M, Mishima H, Ikenaga
M, et al. A case of metastatic gastrointestinal stromal tumor developing a resistance to STI571 (imatinib mesylate). Gan To Kagaku Ryoho
2004;31:1791-4. Japanese.[LinkOut]
- Inage Y, Yamabe K, Yamamoto T, Sato Y, Ishikawa S, Onizuka M, et al.
Resection for pulmonary metastasis of gastrointestinal stromal tumor
of the stomach at 10 years after gastrectomy; report of a case. Kyobu
Geka 2002;55:907-11. Japanese.[LinkOut]
- Ishikawa A, Teratani T, Ono S, Ochiai T, Kakinoki N, Kishimoto Y, et al.
A case of gastrointestinal stromal tumor with liver and bone metastases
effectively treated with radiofrequency ablation and imatinib mesylate.
Nippon Shokakibyo Gakkai Zasshi 2006;103:1274-9. Japanese.[LinkOut]
- Shabahang M, Liv ingstone AS. Cutaneous metastasis from a
gastrointestinal stromal tumor of the stomach: review of literature. Dig
Surg 2002;19:64-5.[LinkOut]
- Anagnostoulis S, Mimidis K, Papadopoulos V, Papa lazarou D,
Argyropoulou P, Iakovidis C, et al. Subcutaneous metastasis from a
gastrointestinal stromal tumor of the stomach: a case report. J BUON
2007;12:549-52.[LinkOut]
- Bara T, Bancu S, Bara T Jr, Muresan M, Bancu L, Azamfirei L, et al.
Gastric stomal tumor with liver and subcutaneous metastasis. Case
report. Chirurgia (Bucur) 2009;104:621-4. Romanian.[LinkOut]
- Hughes B, Yip D, Goldstein D, Waring P, Beshay V, Chong G. Cerebral
relapse of metastatic gastrointestinal stromal tumor during treatment
with imatinib mesylate: Case report. BMC Cancer 2004;4:1-7.[LinkOut]
- Kroep JR, Bovee J, van der Molen AJ, Hogendoorn P, Gelderblom H.
Extra-abdominal subcutaneous metastasis of a gastrointestinal stromal
tumor: report of a case and a review of the literature. J Cutan Pathol
2009;36:565-9.[LinkOut]
- Wang W, Hornick JL, Mallipeddi R, Zelger BG, Rother JD, Yang D, et
al. Cutaneous and subcutaneous metastases of gastrointestinal stromal
tumors: A series of 5 cases with molecular analysis. Am J Dermatopathol
2009;31:297-300.[LinkOut]
- Heinrich MC, Owzar K, Corless CL, Benjamin RS, Bramwell VH,
Demetri GD, et al. Correlation of kinase genotype and clinical outcome
in the North American Intergroup Phase III Trial of imatinib mesylate
for treatment of advanced gastrointestinal stromal tumor: CALGB
150105 Study by Cancer and Leukemia Group B and Southwest
Oncology Group. J Clin Oncol 2008;26:5360-7.[LinkOut]
- Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith
D, et al. Primary and secondary kinase genotypes correlate with
the biological and clinical activity of sunitinib in imatinib-resistant
gastrointestinal stromal tumor. J Clin Oncol 2008;26:5352-9.[LinkOut]
- Fletcher JA, Corless CL, Dimitrijevic S, Von Mehren M, Eisenberg B,
Joensuu H, et al. Mechanisms of resistance to imatinib mesylate (IM)
in advanced gastrointestinal stromal tumor (GIST) [abstract]. Proc Am
Soc Clin Oncol 2003;22:815.
- Mearadji A, den Bakker MA, van Geel AM, Eggermont AM, Sleijfer S,
Verweij J, et al. Decrease of CD117 expression as a possible prognostic
marker for recurrence in the resected specimen after imatinib treatment
in patients with unresectable gastrointestinal stromal tumors: a
clinicopathological analysis. Anticancer Drugs 2008;6:607-12.[LinkOut]
Cite this article as:
Abuzakhm S, Acre-Lara C, Zhao W, Hitchcock C, Mohamed N, Arbogast D, Shah M. Unusual metastases of gastrointestinal stromal tumor and genotypic correlates: Case report and review of the literature. J Gastrointest Oncol. 2011;2(1):45-49. DOI:10.10.3978/j.issn.2078-6891.2011.006
|