Molecular pathology of pancreatic neuroendocrine tumors
Introduction
Pancreatic endocrine tumors (PETs) are rare cancers which account for 1% to 2% of all pancreatic malignancies with approximately 1,000 new cases per year in the United States (1). Epidemiological data show a worldwide increase in the prevalence and incidence of pancreatic neuroendocrine tumors in the past few decades, which is probably due to improved methods of detection of these tumors. PETs originate in islet cells of the endocrine pancreas. There is no gender or age predilection for PETs. The peak incidence for PETs is from age 30 to 60 years, while patients with multiple endocrine neoplasia 1 (MEN1) syndrome have tumors that occur at a younger age.
PETs tend to have an indolent behavior, and long-term survival is common. Five-year survival of PETs is about 55% when the tumors are localized and resected but only about 15% when the tumors are not resectable (2). Overall, PETs still have a much better prognosis than the common exocrine adenocarcinomas of the pancreas (1).
Pancreatic endocrine tumors (PETs) have been a focus of fascination for both pathologists and clinicians for almost a century. Nicholls documented an example of a pancreatic neoplasm in 1902 that was termed an “islet cell adenoma,” and Fabozzi described a biologically malignant counterpart of that lesion the following year (3). Patients can present with symptoms due to hormonal excess or a local mass effect or be asymptomatic (4).
Most PETs are functional, but about 15% are nonfunctional. Because of the presence of several cell types in the pancreatic islets (alpha, beta, delta, PP and Epsilon cells), the term, islet cell tumors, refers to at least five distinct cancers that, when functional, produce unique metabolic and clinical characteristics (4,5). Functional tumors may even be too small to be detected by conventional imaging techniques. The clinical manifestations in functional tumors may result from the distinctive metabolic effects of the polypeptide(s) secreted by the cancer cells rather than from tumor bulk or metastatic disease. The functional tumors, which usually present with symptoms due to hypersecretion of hormone or bioamines, are often classified by the hormone most strongly secreted, for example: Insulinoma (45%), Gastrinoma (20%), Glucagonoma (13%), VIP (vasoactive intestinal peptide)oma (10%), and Somatostatinoma (5%). (I) Insulinoma: hypoglycemia occurs with concurrent elevations of insulin, proinsulin and C peptide (4). (II) Gastrinoma: the excessive gastrin causes Zollinger-Ellison Syndrome (ZES) with peptic ulcers and diarrhea (5). (III) Glucagonoma: the symptoms are not all due to glucagon elevations, and include a rash, sore mouth, altered bowel habits, venous thrombosis, and high blood glucose levels (5). (IV) Somatostatinoma: these rare tumors are associated with elevated blood glucose levels, achlorhydria, cholelithiasis, and diarrhea (5). (V) VIPoma: producing excessive vasoactive intestinal peptide, which may cause profound chronic watery diarrhea and resultant dehydration, hypokalemia, and achlorhydria (WDHA or pancreatic cholera syndrome) (5).
The less common types include ACTHoma, CRHoma, Serotoninoma, Calcitoninoma, GHRHoma, GRFoma, and parathyroid hormone-related peptide tumor.
Nonfunctioning PETs are either an incidental finding or are associated with an expanding mass rather than a hormonal syndrome. Nonfunctional tumors tend to present at later clinical stages with symptoms attributable to mass effect or metastases. Although nonfunctional tumors do not produce specific clinical syndromes, they may secrete inactive amine and peptide products such as neurotensin, alpha-subunit of human chorionic gonadotropin (alpha-hCG), neuron-specific enolase, pancreatic polypeptide (PP) and chromogranin A.
Histopathology findings
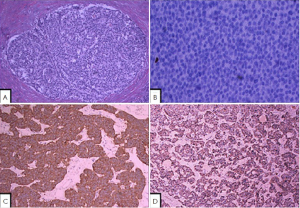
PETs may be either well circumscribed or infiltrative. The cut surface appears red to tan, reflecting the abundant microvasculature, or sometimes yellow because of high lipid content. Morphologically, well-differentiated PETs have characteristic “organoid” arrangements of the tumor cells, with solid, nested, trabecular, or ribbon-like/gyriform, tubuloacinar/psuedoglandular and mixed patterns. The cells are relatively uniform, with round to oval nuclei, coarsely granular and stippled (imparting the classical “salt-and-pepper” appearance) chromatin, and variable from pale to moderately eosinophilic cytoplasm. The cells produce abundant neurosecretory granules, as reflected in the strong and diffuse immunohistochemical expression of neuroendocrine markers such as synaptophysin and chromogranin. Electron microscopy can identify secretory granules. There is usually minimal pleomorphism but less commonly there can be anaplasia, mitotic activity, and necrosis (1).
Generally, the histologic features of the tumor do not correlate with anatomic location or hormone production, but there are exceptions: amyloid deposition (insulin-associated peptide) often indicates an insulin-secreting PET, and glandular architecture with abundant psammoma body formation is usually seen in periampullary somatostatin-secreting PETs (1).
The morphologic spectrum of these tumors can be variable, and the pathologic differential diagnosis includes chronic pancreatitis with neuroendocrine hyperplasia, poorly differentiated ductal adenocarcinoma, solid pseudopapillary tumor, acinar cell carcinoma, and pancreatoblastoma (6). However, serologic or immunohistochemical evidence for elevated hormones may be identified for PETs. PETs show tissue immunoreactivity for markers of neuroendocrine differentiation (chromogranins, synaptophysin, neuron-specific enolase, PGP9.5 and CD56) and may secrete various peptides and hormones. Expression of peptides such as insulin, glucagon, PP, somatastain, gastrin or VIP is common, and most functional PETs can be shown to produce the appropriate peptide by immunohistochemistry. In addition, minor cell populations producing a variety of other peptides are commonly detectable. Neuroendocrine secretory protein-55, a member of the chromogranin family, is seen in pancreatic endocrine tumors but not intestinal NETs. It is important to be aware of the unusual morphologic variants of pancreatic endocrine tumors, and select immunohistochemical markers can help avoid misdiagnosis (3).
The mitotic rate is an important measure of aggressiveness in PETs. Well-differentiated PETs are defined to have less than 20 mitotic figures per 10 high power fields (hpf); neoplasms with 20 or more mitoses per 10 hpf are considered poorly differentiated (high grade) neuroendocrine carcinomas (Table 1). In many PETs, mitotic figures are nearly undetectable, a search of 50 hpf (or more) may be required for a single mitotic figure. Some PETs have a higher proliferative rate; and the finding of 2 or more mitotic figures per 10 hpf places a PET in a worse prognostic category. Necrosis is also variably present; most commonly it is accompanied with an increased in proliferative rrate, thus signifing a more aggressive PET (5) (Figure 1).

Full table
Pathogenesis of PETs
Most pancreatic neuroendocrine tumors occur sporadically (90%). However, they may be part of hereditary syndromes: multiple endocrine neoplasia type 1 (MEN1 syndrome), von Hippel-Lindau disease (VHL), von Recklinghausen’s disease or neurofibromatosis type 1 (NF-1), and tuberous sclerosis (TSC) (5). In these cases, the underling genetic abnormalities play a significant role in the development of PETs which are often found to be mutlifocal. The pathological features of familial/hereditary PETs are generally similar to the sporadic form, although PETs arising in VHL syndrome patients may have clear cell features (6).
Germline loss-of-function MEN-1 mutation leads to the formation of numerous microadenomas, mostly resulting in non-functional PETs and insulinomas (7). NF-1 or TSC1/2 mutations result in loss of function of their protein products neurofibromin and tuberin, respectively. Notably, the intact proteins suppress the function of a common target, namely mTOR (mammalian target of rapamycin) (7). Furthermore, hypoxia-induced factor (HIF)-dependent mTOR activation links disturbed mTOR signaling to VHL disease (8,9). mTOR is a key regulator of cell growth and integrates a wide variety of cellular inputs, such as growth factors, nutrients, energy status and hypoxia-induced stress, thus, it is a good therapeutic target for PETs.
Somatic MEN1 gene mutations accompanied by a loss of the wild-type allele are demonstrated in 10-27% of insulinomas and 39-45% of gastrinomas. The rate of 11q13 loss of heterozygosity (LOH) in sporadic PETs is about 46%, and LOH is not always accompanied by somatic mutation, therefore, other mechanisms of MEN1 gene inactivation or other genes may play a role in sporadic tumor development. Studies indicate that additional onco/suppressor genes may reside at 11q distal to the MEN1 gene and may play a role in the pathogenesis of PETs (10).
Sporadic endocrine pancreatic tumors: molecular genetics and pathobiology genome-wide analyses by comparative genomic hybridization (CGH) indicate that the chromosomal losses occur slightly more frequently than gains, whereas amplifications are uncommon. Losses of chromosome 1 and 11q as well as gains of 9q appear to be early events in the development of pancreatic tumors (10,11). These findings point towards a tumor suppressor pathway and chromosomal instability as important mechanisms associated with malignancy in pancreatic endocrine tumors. Gains of chromosome 4 and losses of 6q were observed in about 50% of functioning tumors, the majority being insulinomas, with a size less than 2 cm (12). Recent studies using genome-wide single nucleotide polymorphism (SNP) analysis showed that about 30-40% of pancreatic endocrine tumors had high genetic imbalances defined by chromosomal aberrations (13,14). Homozygous deletion or hypermethylation of p16/MTS1 or a deletion of the p16INK4a tumor suppressor gene on chromosome 9p21 was demonstrated in sporadic gastrinomas, but not in insulinomas. Both benign and malignant insulinomas demonstrated high LOH rates for markers on chromosome 22q (93%) (15). Cyclin D1 overexpression was observed by both immunohistochemistry and northern blot analysis in 43% of PETs (16). High-grade PETs share a large fraction of gene abnormalities with conventional cancers, the most frequent abnormality being in the cell-cycle key regulatory gene TP53. In summary, the data suggest that multiple genetic defects may accumulate and result in PETs progression and malignancy. Molecular genetic tests are relevant to the pathogenesis, however, these tests are currently not useful in the diagnostic process (15). The epigenetic modifications and differential microRNA-expression mechanistically involved in the dysregulated signaling pathways of PETs are under further investigation (17,18).
Classification and grading of PETs
The classification of PETs has been controversial, and prognosis is difficult to predict, but important features include metastasis and invasion of adjacent structures (3,19).
In the past, two grading schemes have been accepted for pancreatic endocrine tumors (WHO and MSKS), each places a given tumor into categories depending on well-defined histological features: size, lymphovascular invasion, mitotic counts, Ki-67 labelling index, invasion of adjacent organs, presence of metastases and whether the tumor produces hormones (5). Whichever system is chosen, it is clear that almost all of these tumors have the potential to metastasize, even after many years.
- Well-differentiated pancreatic endocrine tumor
- “Benign” behavior: confined to pancreas, no vascular or perineural invasion, <2 cm, and <2 MF/10 HPF
- Uncertain behavior: confined to pancreas, vascular and/or perineural invasion, >2 cm, or 2-10 MF/10 HPF
- Well-differentiated pancreatic endocrine carcinoma
- Gross local invasion or metastases
- Low-grade pancreatic endocrine neoplasm
- No necrosis and <2 MF/50 HPF
- Intermediate-grade pancreatic endocrine neoplasm
- Necrosis or between 2 to 50 MF/50 HPF
Since the distinction between benign and malignant PETs can be difficult, some authors have attempted to define prognostic factors without designating tumors as benign. Because of the difficulty in determining which PETs are malignant, many pathologists use the term carcinoma for all PETs, or malignant. The WHO 2010 neuroendocrine neoplasm classification has introduced grading and staging; low to intermediate grade tumors are defined as neuroendocrine tumors (previously carcinoids) whereas high-grade carcinomas are termed neuroendocrine carcinomas (20). Pathologists are becoming to accept the WHO (2010) grading system, adopted from the European Neuroendocrine Tumor Society (ENTS) proposal for grading all gastoenteropancreatic neuroendocrine tumors (21). In addition to the 3-tier grade-based classification, TNM staging of PETs can now be performed (AJCC/UICC) using the same parameters applied for exocrine type carcinomas of the pancreas (22).
The newly updated WHO 2010 classification scheme uses a proliferation-based grading system together with the classical histopathological diagnostic criteria for PETs (Table 2) (19). In the WHO 2010 classification, the malignant potential of pancreatic neuroendocrine neoplasms is acknowledged and enforced. The fact is that PETs are often malignant because they are metastatic at diagnosis, or at least have the potential to metastasize in a size-dependent fashion. The new classification aims to standardize current diagnostic and management procedures and enable systematic and prognostically relevant patient stratification. PETs are graded into 1 of 3 tiers, either as well-differentiated neuroendocrine tumors or poorly-differentiated neuroendocrine carcinomas, on the basis of stage-pertinent features such as proven invasion or metastasis (5).
The grading system still remains controversial, but clear signs of malignancy include metastasis and local or extrapancreatic invasion. Other characteristics that appear helpful in determining prognosis are tumor size and functional status, necrosis, mitotic activity, perineural invasion and angioinvasion, and possibly CD44 isoform upregulated expression and cytokeratin 19 immunostaining (5,23). Peptide production detected in the serum or by immunohistochemistry is not a prognostic factor for nonfunctional PETs (3). Nuclear pleomorphism is also not a useful predictor; however some studies have demonstrated a correlation between overall nuclear grade and prognosis (24). The TNM system has proved to be highly predictive of patient outcome and is easy to combine with histologic and clinicopathologic parameters to classify pancreatic endocrine tumors into groups of increasing malignant potential (19,22).
Treatment
In the past, treatment options for PETs have been limited, with hormonal treatment with octreotide (somatostatin analogues) as the primary therapeutic approach. Some PETs possess especially strong hormone receptors, such as somatostatin receptors and uptake hormones strongly (5). This avidity can assist in diagnosis and may make some tumors vulnerable to hormone targeted therapies.
Although the optimal clinical management of PNETs involves a multidisciplinary approach, surgery remains the only curative treatment for early-stage disease. The surgical treatment continues to evolve for PETs, but the best outcome occurs in those treated with total tumor resection. Surgery may also have a role in patients with advanced-stage disease, including those with hepatic metastases (25). Alternative therapeutic approaches applied to PETs, including chemotherapy, radiofrequency ablation, transarterial chemoembolization, biotherapy, polypeptide radionuclide receptor therapy, antiangiogenic therapy, and selective internal radiotherapy (7). Chemotherapeutic agents have been used with limited efficacy (less effective in well-differentiated tumors). Several agents have shown activity and combining several thearpies, particularly doxorubicin with streptozocin, is often more effective (26). Although marginally effective in well-differentiated PETs, cisplatin with etoposide is active in poorly-differentiated neuroendocrine cancers (5,26).
Targeted therapy has a clear role as these tumors do overexpress receptors for EGF, PDGF, IGF-1, and VEGF. Recent studies demonstrate PI3K/Akt/mTOR pathway is involved in the pathogenesis of PETs (8,9). Based on the phase III clinical trials data, mTOR inhibitor (Everolimus) significantly improved progression-free survival among patients with progressive advanced pancreatic neuroendocrine tumors as compared with placebo (9). This targeted chemotherapy agents have been approved by FDA in patients with progressive unresectable, locally advanced or metastatic pancreatic neuroendocrine tumors. The combination of an mTOR inhibitor and a VEGF inhibitor has also showed promising results (8).
Conclusions
In summary, pancreatic neuroendocrine tumors are generally indolent neoplasms, even though the majority do present at an advanced stage. Once PETs is suspected based on the histologic features, immunohistochemistry plays a critical role to confirm the diagnosis. The 2010 WHO classification of tumors of the digestive system introduces grading and staging tools for pancreatic neuroendocrine neoplasms. A carcinoid is now defined as a grade 1 or 2 neuroendocrine tumor and grade 3, small-cell or large-cell carcinomas are defined as neuroendocrine carcinomas. Besides surgery and somatostatin analogues treatment, the emerging compounds including chemotherapeutic agents and target therapies may provide new hope for patients with PETs.
Acknowledgments
We acknowledge the support provided by the UC Davis Health System National Board of Advisors Vision grant awarded to M.C.
Disclosure: The authors declare no confict of interest.
References
- Heitz PU KP, Perren A, Klimstra D, et al. Tumors of the endocrine pancreas. In: DeLellis RA LR, Heitz PU, Eng C eds. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: France IARC Press, 2004:175-208.
- Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 2010;23:824-33. [PubMed]
- Wick MR, Graeme-Cook FM. Pancreatic neuroendocrine neoplasms: a current summary of diagnostic, prognostic, and differential diagnostic information. Am J Clin Pathol 2001;115:S28-45. [PubMed]
- Davì MV, Falconi M. Pancreas: Insulinoma--new insights into an old disease. Nat Rev Endocrinol 2009;5:300-2. [PubMed]
- Rindi G, Wiedenmann B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol 2011;8:54-64. [PubMed]
- Frankel WL. Update on pancreatic endocrine tumors. Arch Pathol Lab Med 2006;130:963-6. [PubMed]
- Ehehalt F, Saeger HD, Schmidt CM, et al. Neuroendocrine tumors of the pancreas. Oncologist 2009;14:456-67. [PubMed]
- Wiedenmann B, Pavel M, Kos-Kudla B. From targets to treatments: a review of molecular targets in pancreatic neuroendocrine tumors. Neuroendocrinology 2011;94:177-90. [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [PubMed]
- Lubensky IA, Zhuang Z. Molecular genetic events in gastrointestinal and pancreatic neuroendocrine tumors. Endocr Pathol 2007;18:156-62. [PubMed]
- Bloomston M, Durkin A, Yang I, et al. Identification of molecular markers specific for pancreatic neuroendocrine tumors by genetic profiling of core biopsies. Ann Surg Oncol 2004;11:413-9. [PubMed]
- Arnold CN, Sosnowski A, Schmitt-Gräff A, et al. Analysis of molecular pathways in sporadic neuroendocrine tumors of the gastro-entero-pancreatic system. Int J Cancer 2007;120:2157-64. [PubMed]
- Kim H, Nagano Y, Choi IS, et al. Allelic alterations in well-differentiated neuroendocrine tumors (carcinoid tumors) identified by genome-wide single nucleotide polymorphism analysis and comparison with pancreatic endocrine tumors. Genes Chromosomes Cancer 2008;47:84-92.
- Nagano Y, Kim H, Zhang L, et al. Allelic alterations in pancreatic endocrine tumors identified by genome-wide single nucleotide polymorphism analysis. Endocr Relat Cancer 2007;14:483-92.
- Oberg K. Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors). Curr Opin Endocrinol Diabetes Obes 2009;16:72-8. [PubMed]
- Chung DC, Brown SB, Graeme-Cook F, et al. Overexpression of cyclin D1 occurs frequently in human pancreatic endocrine tumors. J Clin Endocrinol Metab 2000;85:4373-8. [PubMed]
- House MG, Herman JG, Guo MZ, et al. Aberrant hypermethylation of tumor suppressor genes in pancreatic endocrine neoplasms. Ann Surg 2003;238:423-31; discussion 431-2. [PubMed]
- Roldo C, Missiaglia E, Hagan JP, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol 2006;24:4677-84. [PubMed]
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-12. [PubMed]
- Alsamarai S, Libutti SK, Saif MW. Updates in pancreatic neuroendocrine carcinoma. Highlights from the “2010 ASCO Annual Meeting”. Chicago, IL, USA. June 4-8, 2010. JOP 2010;11:336-340. [PubMed]
- Boudreaux JP, Klimstra DS, Hassan MM, et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas 2010;39:753-66. [PubMed]
- Exocrine and endocrine pancreas. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010:241-9.
- Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol 2002;20:2633-42. [PubMed]
- Zee SY, Hochwald SN, Conlon KC, et al. Pleomorphic pancreatic endocrine neoplasms: a variant commonly confused with adenocarcinoma. Am J Surg Pathol 2005;29:1194-200. [PubMed]
- Teh SH, Deveney C, Sheppard BC. Aggressive pancreatic resection for primary pancreatic neuroendocrine tumor: is it justifiable? Am J Surg 2007;193:610-3; discussion 613. [PubMed]
- Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol 2011;29:934-43. [PubMed]