Functioning gangliocytic paraganglioma of the ampulla: clinicopathological correlations and cytologic features
Introduction
The preoperative diagnosis of ampullary tumor becomes increasingly practical and important with the widespread use of endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA). The most common ampullary/periampullary tumor is adenocarcinoma followed by adenoma and neuroendocrine tumor (1). Gangliocytic paraganglioma (GP) is a rare neuroendocrine tumor that is almost exclusively found in the ampulla, though rare cases involving nasopharynx, lung, and other parts of the digestive tract have been reported (2-4). GP is generally considered to be benign, but up to 5% of cases demonstrate malignant behavior such as lymph node spread and distant metastasis. It is believed to be a nonfunctioning neuroendocrine tumor, except for one case report of a corticotropin-producing GP arising in the lung (4). We herein present a case of a functionally active ampullary GP with lymph node metastasis. The cytologic features that help make the diagnosis on FNA are also discussed.
Case presentation
Clinical history
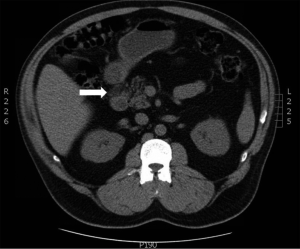
A 45-year-old Hispanic male presented with upper abdominal cramps, vomiting and diarrhea, episodic melena, and weight loss for 4 months. Medical history was positive for diabetes mellitus of unknown duration. Computed tomography (CT) revealed a 2.5 cm mass in the second portion of duodenum and regional lymphadenopathy (Figure 1). No other masses were identified. Laboratory tests showed elevated serum chromogranin A (135 ng/mL, reference <93 ng/mL), serotonin (569 ng/mL, reference ≤230 ng/mL), and hemoglobin A1c (HbA1c) (8.2%, reference 4.5-6.2%). Carcinoembryonic antigen (CEA), amylase, alkaline phosphatase, and bilirubin were within normal range. The clinical diagnosis of carcinoid tumor was made. The patient subsequently underwent EUS, which confirmed an ampullary mass that did not appear to invade through the muscularis propria of the duodenal wall. An EUS-guided FNA was subsequently performed.
Cytological findings
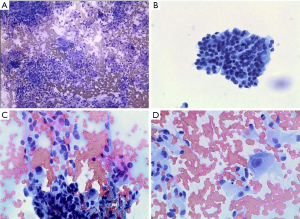
Review of air dried Diff Quik and alcohol fixed Papanicolaou-stained smears showed a highly cellular yield with a clean background. No tumor diathesis was seen (Figure 2A). The dominant cell type was a small- to medium-sized epithelioid cell with cells arranged in clusters. Other cell populations included interspersed spindle cells and occasional large ganglion-like cells (Figure 2A). On higher power, the epithelioid cells were arranged in syncytia and had indistinct cytoplasmic borders. Vague rosettes or acinar patterns were noted. The epithelioid cells had scant to moderate amounts of granular cytoplasm, round to oval nuclei and low nuclear to cytoplasmic ratios. The nuclei had smooth borders and coarsely granular chromatin. The nuclei were often eccentrically situated, giving the cells a plasmacytoid appearance (Figure 2B). Some of the epithelioid cell clusters had delicate capillary networks and bland spindle cells at the periphery (Figure 2C). The ganglion-like cells showed abundant cytoplasm, large round nuclei with smooth nuclear contours, homogeneous fine chromatin, and prominent nucleoli (Figure 2D). Mitotic figures were not seen. No amyloid-like material was identified.
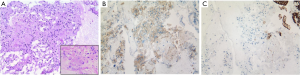
The cell block consisted of predominantly epithelioid cells, interspersed spindle cells, occasional ganglion-like cells, and contaminating normal intestinal epithelium (Figure 3A). Immunostaining performed on the cell block showed the tumor cells were positive for synaptophysin (Figure 3B), and negative for pancytokeratin (pan-CK) (Figure 3C), smooth muscle actin and CD117.
Histopathological findings
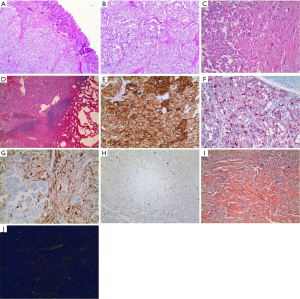
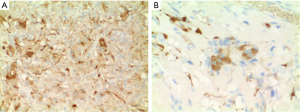
The patient underwent ampullectomy with periduodenal/retropancreatic lymph node dissection. Gross examination revealed a well-circumscribed, pedunculated, white-yellow nodule at the ampulla, measuring 1.5 cm × 1.5 cm × 1.3 cm in size. Microscopic examination of hematoxylin and eosin (H & E) stained slides showed a submucosal, nonencapsulated, multilobulated mass (Figure 4A). The epithelioid cells were arranged in nests or trabeculae. The nests were surrounded by spindle cells and delicate strands of fibrovascular stroma, consistent with a Zellballen pattern (Figure 4B). Scattered large ganglion-like cells were noted between the epithelioid cell nests (Figure 4C). One periduodenal lymph node had metastatic tumor (Figure 4D). Immunohistochemically, the epithelioid cells showed diffuse strong reactivity for CD56 (Figure 4E) and synaptophysin, and focal positivity for chromogranin. S-100 staining highlighted spindled sustentacular cells (Figure 4F). Neurofilament protein (NFP) was expressed in the ganglion-like cells and axon-like cell extensions (Figure 4G). The tumor cells were not mitotically active, with Ki-67 labeling indices no more than 10% (Figure 4H). Amorphous and eosinophilic extracellular material noted on H & E was positive with Congo red stain (Figure 4I) and displayed apple-green birefringence under polarized light (Figure 4J), typical for amyloid. Both epithelioid cells and ganglion-like cells were positive for somatostatin (Figure 5). Serotonin staining was negative.
Additional intraoperative findings
There were two incidental intraoperative findings: a 1.5 cm subserosal mass on the anterior stomach, and a very small, contracted gallbladder containing a large gallstone. The pathological diagnoses were gastrointestinal stromal tumor (GIST) and chronic calculous cholecystitis, respectively.
Clinical follow-up
During his post-operative hospital stay for 7 days, the patient was euglycemic with no need for antihyperglycemic medications. CT scan 3 months post-surgery showed lymphadenopathy surrounding the duodenum and portal vein, suspicious for residual/recurrent/metastatic disease. In the interim, control of his blood glucose level became poor, and he became symptomatic with flushing and hot flashes. The patient was then lost to follow-up.
Discussion
In 2013, Dustin et al. published the first cytology case report of GP (5). The most characteristic feature described was the presence of three cell populations of epithelioid, ganglion-like, and spindle cells, which had been emphasized in the numerous histopathological case reports of this entity. Herein, we would like to discuss other noteworthy cytologic features and differential diagnoses.
First of all, GP is a submucosal lesion. So, FNA material often contains contaminating intestinal epithelium, which may appear either normal or reactive. That being said, GP itself can have glandular or tubular components (6-9). Rarely, it has been reported to coexist with invasive duodenal adenocarcinoma (10). In either circumstance, GP on FNA may be confused with mixed adenoneuroendocrine carcinoma of the ampulla (1). Noting the aforementioned tricellular composition, FNA specimens of GP usually demonstrate a clean background lacking tumor diathesis. Cytologically, the malignant GP cases may have mild to moderate nuclear pleomorphism and mitoses, but necrosis is usually absent (9,11).
Another supportive though nonspecific cytologic feature of GP is a delicate capillary network on smear. Paraganglioma is known to have delicate fibrovascular stroma which wraps tumor cell nests, giving the characteristic “Zellballen” appearance. GP shows similar features.
One interesting morphologic feature is the presence in some cases of stromal amyloid, which can be easily overlooked or mistaken for fibrin or collagen bundles on FNA smears or cell block. Amyloid appears as dark-blue to purple clumps of acellular material on air-dried Romanowsky (e.g., Diff Quik) stained smears and as cyanophilic to orangophilic acellular material on alcohol-fixed Papanicolaou stained smears (12,13). When in doubt, a Congo red stain should be done on either smears or cell block to confirm amyloid. It is important to note that GP can show immunoreactivity for calcitonin (14,15), so this stain should not be used to differentiate it from metastatic medullary thyroid carcinoma, which will also be positive.
Lastly, cytokeratin (CK) staining has limited utility in confirming the diagnosis of GP. In the case reported by Dustin (5), epithelioid cells were negative for CK7 and CK20, but positive for pan-CK. In our case, epithelioid cells were negative for pan-CK. In the 51-case series reported by Burke and Helwig, CK was positive in 52% of the cases (16). Some argue that CAM5.2 might give a higher positive rate of CK (17). However, limited data show that some of the cases are truly CK negative, even when both CAM5.2 and AE1/AE3 are employed (18).
Clinically, GP is generally considered nonfunctioning and therefore not associated with endocrine syndromes (16). What makes our case unique is the constellation of ampullary neuroendocrine tumor, flushing, diarrhea, weight loss, diabetes mellitus, and cholelithiasis. All the features, except flushing, are suggestive of somatostatinoma syndrome (19), while his flushing, diarrhea and elevated serum serotonin level led to the pre-operative clinical diagnosis of carcinoid tumor. Both epithelioid cells and ganglion-like cells of GP are known to make somatostatin (8,9,14-16,20-23), though it may not always get secreted to cause endocrine symptoms. In keeping with the literature, the present case shows somatostatin immune-reactivity (polyclonal, Ventana Medical Systems, Catalog number 760-2667) in epithelioid cells and ganglion-like cells. Though serum somatostatin was not measured in our patient, the elevated preoperative serum levels of serotonin and chromogranin A are evidence of active secretion by the tumor. The discrepancy between elevated serum serotonin and negative immunostaining for serotonin in our case might be due to the different sensitivities of the methods, since there was no lesion elsewhere on the preoperative staging workup to explain the elevated serum serotonin. Serum serotonin was measured at Mayo Clinic Medical Laboratories by liquid chromatography-tandem mass spectrometry which has high sensitivity and specificity, whereas the serotonin antibody (clone 5HT-H209, Dako, Catalog number M0758) used in our immunostaining is a monoclonal antibody which has excellent specificity but limited sensitivity. Moreover, optimal staining of this antibody on formalin-fixed paraffin-embedded tissue is highly dependent on the method of epitope retrieval, per the specification sheet by the manufacturer. In fact, serotonin expression in GP has been well documented (14,16,22,24). The regional lymphadenopathy suspicious for residual/recurrent/metastatic disease 3 months after surgery plus accompanying symptoms of flushing, hot flashes and worsening hyperglycemia are potentially additional evidence of a functioning tumor. Unfortunately, the patient was then lost to follow-up and transferred his care to another facility. Per communication with the current treating physician, the patient had a negative octreotide scan and urinary 5-HIAA on follow-up. To better characterize the suspicious lesion seen on CT, a positron emission tomography (PET)-CT was done but the result was not available to us. It is known that neuroendocrine tumors that secrete somatostatin may be missed on octreotide scan due to the presence of unlabeled somatostatin. Therefore, negative octreotide scan might be further evidence of a functioning tumor in this case. Unfortunately, it is difficult to prove since a preoperative octreotide scan was not performed. Regarding the negative urinary 5-HIAA, it is known that 15-70% of functioning neuroendocrine tumors are missed with urinary 5-HIAA test. Serum serotonin assay is a better way to detect these tumors.
A literature search yielded only one other case of a functioning GP (4). The patient presented with Cushing’s syndrome. Workup revealed a corticotropin-producing pulmonary GP. Cortisol and corticotropin levels dropped after the surgery. Our case is the second reported case of a functioning GP, and the first reported functioning ampullary GP. Since GP arises almost exclusively in the ampulla of the duodenum, we consider our case report to be a significant contribution to the literature regarding these tumors. It has been reported that, after complete resection of a somatostatin-producing tumor, the patient may become euglycemic (25). Indeed, our patient was euglycemic during his postoperative hospital stay. Besides somatostatin, other possible contributors to our patient’s hyperglycemia include glucagon, though glucagon expression was not confirmed in our patient. It is well established that glucagon often plays an important role in endocrine tumor-associated hyperglycemia. Interestingly, glucagon immunoreactivity in GP has been reported by several groups independently (10,26,27).
Both GP and GIST have been weakly associated with neurofibromatosis type 1 (NF-1) (24,28). NF-1, also known as von Recklinghausen disease, is caused by inherited or sporadic mutations of the tumor suppressor gene NF-1. Since our patient has no clinical evidence of NF-1, his GP and GIST are likely coincidental. However, the possibility of a common molecular pathway being responsible for both entities in our case, for instance, up-regulation of RAS pathway due to somatic NF-1 mutation, cannot be excluded without molecular genetic study.
Regarding treatment, surgery is the mainstay. Local or Whipple resection with or without lymph node dissection is generally curative, with no recurrence on follow-up up to 25 years (22). Even for those cases with lymph node spread, follow-up up to 91 months showed no evidence of disease. We advocate increased awareness of the functioning potential of GP so that neuroendocrine symptoms, if present, are managed appropriately.
Acknowledgements
The authors would like to thank Dr. Douglas Hegstad at Department of Medicine, Loma Linda University Medical Center for his input on the potential role of glucagon in hyperglycemia of patients with gangliocytic paraganglioma.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhang L, DeMay RM. Cytological features of mixed adenoneuroendocrine carcinoma of the ampulla: two case reports with review of literature. Diagn Cytopathol 2014;42:1075-84. [PubMed]
- Sinkre P, Lindberg G, Albores-Saavedra J. Nasopharyngeal gangliocytic paraganglioma. Arch Pathol Lab Med 2001;125:1098-100. [PubMed]
- Hironaka M, Fukayama M, Takayashiki N, et al. Pulmonary gangliocytic paraganglioma: case report and comparative immunohistochemical study of related neuroendocrine neoplasms. Am J Surg Pathol 2001;25:688-93. [PubMed]
- Paláu MA, Merino MJ, Quezado M. Corticotropin-producing pulmonary gangliocytic paraganglioma associated with Cushing's syndrome. Hum Pathol 2006;37:623-6. [PubMed]
- Dustin SM, Atkins KA, Shami VM, et al. The cytologic diagnosis of gangliocytic paraganglioma: a case report. Diagn Cytopathol 2013;41:650-3. [PubMed]
- Ogata S, Horio T, Sugiura Y, et al. Duodenal gangliocytic paraganglioma with regional lymph node metastasis and a glandular component. Pathol Int 2011;61:104-7. [PubMed]
- Shi H, Han J, Liu N, et al. A gangliocytic patially glandular paraganglioma with lymph node metastasis. Diagn Pathol 2014;9:63. [PubMed]
- Inai K, Kobuke T, Yonehara S, et al. Duodenal gangliocytic paraganglioma with lymph node metastasis in a 17-year-old boy. Cancer 1989;63:2540-5. [PubMed]
- Rowsell C, Coburn N, Chetty R. Gangliocytic paraganglioma: a rare case with metastases of all 3 elements to liver and lymph nodes. Ann Diagn Pathol 2011;15:467-71. [PubMed]
- Anders KH, Glasgow BJ, Lewin KJ. Gangliocytic paraganglioma associated with duodenal adenocarcinoma. Case report with immunohistochemical evaluation. Arch Pathol Lab Med 1987;111:49-52. [PubMed]
- Witkiewicz A, Galler A, Yeo CJ, et al. Gangliocytic paraganglioma: case report and review of the literature. J Gastrointest Surg 2007;11:1351-4. [PubMed]
- Sahoo S, Reeves W, DeMay RM. Amyloid tumor: a clinical and cytomorphologic study. Diagn Cytopathol 2003;28:325-8. [PubMed]
- Michael CW, Naylor B. Amyloid in cytologic specimens. Differential diagnosis and diagnostic pitfalls. Acta Cytol 1999;43:746-55. [PubMed]
- Guarda LA, Ordonez NG, del Junco GW, et al. Gangliocytic paraganglioma of the duodenum: an immunocytochemical study. Am J Gastroenterol 1983;78:794-8. [PubMed]
- Watanabe K, Hasegawa H, Sakuma H, et al. Two cases of duodenal gangliocytic paraganglioma: immunocytochemical characteristics. Fukushima J Med Sci 1995;41:141-52. [PubMed]
- Burke AP, Helwig EB. Gangliocytic paraganglioma. Am J Clin Pathol 1989;92:1-9. [PubMed]
- Weinrach DM, Wang KL, Blum MG, et al. Multifocal presentation of gangliocytic paraganglioma in the mediastinum and esophagus. Hum Pathol 2004;35:1288-91. [PubMed]
- Abdelbaqi MQ, Tahmasbi M, Ghayouri M. Gangliocytic paraganglioma of the appendix with features suggestive of malignancy, a rare case report and review of the literature. Int J Clin Exp Pathol 2013;6:1948-52. [PubMed]
- Krejs GJ, Orci L, Conlon JM, et al. Somatostatinoma syndrome. Biochemical, morphologic and clinical features. N Engl J Med 1979;301:285-92. [PubMed]
- Barbareschi M, Frigo B, Aldovini D, et al. Duodenal gangliocytic paraganglioma. Report of a case and review of the literature. Virchows Arch A Pathol Anat Histopathol 1989;416:81-9. [PubMed]
- Garbrecht N, Anlauf M, Schmitt A, et al. Somatostatin-producing neuroendocrine tumors of the duodenum and pancreas: incidence, types, biological behavior, association with inherited syndromes, and functional activity. Endocr Relat Cancer 2008;15:229-41. [PubMed]
- Scheithauer BW, Nora FE, LeChago J, et al. Duodenal gangliocytic paraganglioma. Clinicopathologic and immunocytochemical study of 11 cases. Am J Clin Pathol 1986;86:559-65. [PubMed]
- Hamid QA, Bishop AE, Rode J, et al. Duodenal gangliocytic paragangliomas: a study of 10 cases with immunocytochemical neuroendocrine markers. Hum Pathol 1986;17:1151-7. [PubMed]
- Stephens M, Williams GT, Jasani B, et al. Synchronous duodenal neuroendocrine tumours in von Recklinghausen’s disease--a case report of co-existing gangliocytic paraganglioma and somatostatin-rich glandular carcinoid. Histopathology 1987;11:1331-40. [PubMed]
- Ganda OP, Weir GC, Soeldner JS, et al. “Somatostatinoma”: a somatostatin-containing tumor of the endocrine pancreas. N Engl J Med 1977;296:963-7. [PubMed]
- Grouls V, Stein U, Vogel J. Juxtapapillary gangliocytic paraganglioma of the duodenum. Dtsch Med Wochenschr 1989;114:584-8. [PubMed]
- Perrone T, Sibley RK, Rosai J. Duodenal gangliocytic paraganglioma. An immunohistochemical and ultrastructural study and a hypothesis concerning its origin. Am J Surg Pathol 1985;9:31-41. [PubMed]
- Castoldi L, De Rai P, Marini A, et al. Neurofibromatosis-1 and ampullary gangliocytic paraganglioma causing biliary and pancreatic obstruction. Int J Gastrointest Cancer 2001;29:93-8. [PubMed]


