Irreversible electroporation of locally advanced pancreatic neck/body adenocarcinoma
Introduction
Locally advanced stage 3 pancreatic adenocarcinoma has been reported to represent nearly 50% of the 40,000 new patients diagnosed with advanced cancer (1). Outcomes in these rare patients with stage 3 pancreatic neck lesions that undergo resection are still poor. Post-resection 5-year survival has been reported at 6.8% and the median survival after resection has been reported to be 10.6 months (2). This poor prognosis even in the resected patients has historically diminished enthusiasm for aggressive surgical resection (3) in certain subset of patients.
Irreversible electroporation (IRE) is a new technique that utilizes short (70 to 90 us), high-voltage (1,500 to 2,000 volts/cm) pulses to the tissue (4-7) to permeabilize the cell membranes. IRE uses a non-thermal-based (less than 54 degrees for 10 sec) method of action and can be used to treat around vital structures such as the urethra, larger blood vessels, and nerves (5). IRE for locally advanced pancreatic neck/body cancers as a surgical palliative technique in has been reported, the standardization of its utilization has not been thoroughly described. We have recently published our findings regarding the safety of IRE in the pancreas (8). Similarly we have also recently published superior survival rates with the use of IRE in combination with standard chemotherapy and/or chemo-radiation therapy when compared to standard of care chemotherapy or chemoradiation therapy (9). We have recently published the optimal technique for the use of IRE on locally advanced pancreatic cancer of the pancreatic head (10).
This article describes our preferred method for the utilization of open IRE of patients with locally advanced pancreatic adenocarcinoma of the body/neck.
Methods
As reported previously (8-10), our standard work-up for patients with locally advanced pancreatic adenocarcinoma, has been described previously, but includes a 3-phase CT scan with pancreatic protocol with 2.0 mm cuts or less at the time of diagnosis, which allows us to appropriately diagnose and stage patients with locally advanced pancreatic adenocarcinoma (11,12). Laboratory work-up is also performed to ensure appropriate hematologic as well as CA19-9 evaluation. We recommend a staging/diagnostic laparoscopy be performed at the time of diagnosis so that peritoneal washings can be obtained in order to rule out small occult metastases that are not present CT scan. We recommend the use of induction chemotherapy of either a Gemzar-based or FOLFIRINOX-based chemotherapy based on the patient’s age and performance status for at least three to four months in duration (10). After induction chemotherapy, repeat high-quality 3-phase CT scan, and also obtain hematologic and serologic markers to ensure locally advanced non-metastatic pancreatic adenocarcinoma still exists. The key goal of this repeat imaging is to ensure that metastatic disease has not occurred, since it is uncommon for a pancreatic cancer to truly respond to induction therapy (chemotherapy alone or chemo-radiation therapy) based on established response evaluation criteria in solid tumors (RECIST) criteria (i.e., reduction in size of >30% of the longest diameter). As long as the patient has not developed metastatic disease and the maximum axial diameter is not above 3.5 cm after induction therapy, then we do proceed with IRE therapy.
Once this is confirmed, approximately two to four weeks after the last dose of chemotherapy open IRE to the pancreatic tumor primary is performed. Optimal inclusion of patients who are appropriate for IRE should include tumor sizes that are 3.0 to 3.5 cm in maximum diameter. Given that Pancreatic neck/mid body tumors rarely present with jaundice the decision making for biliary stenting is not relevant to this disease location. In either case as we have recently published, removal of metal stents is critical to patient outcomes (13). Given that any type of metal is conductive, it has been demonstrated in our large animal model that these metal stents lead to significant deflection of energy, which can lead to incomplete ablation, high current conditions, and possible thermal injury since the degree of deflection is not consistent based on the location of the metal, the probe exposure and the fibrotic nature of the tissue to be electroporated (13).
Operative description
Abdominal approach
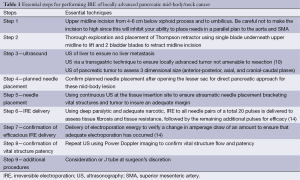
All procedures are performed with general anesthesia through midline incision (Table 1). I prefer a midline (defined as 60% of incision above the umbilicus and 40% below) so that the IRE needles can be placed on as parallel a plan as possible to the aorta based on the tumor infiltration. The safest method for IRE needle placement is in a caudal-to-cranial approach, so that needle placement can be tracked with ultrasound throughout needle placement. Any occult solid organ liver metastases as well as peritoneal or mesenteric metastases are ruled out before proceeding with IRE of any pancreatic lesion. I strongly recommend not using this device/procedure for anyone with stage 4 disease as well as any patient who is chemo-naïve who is found at the time of exploration to have locally advanced stage 3 disease. This therapy should not be used as a bail out for patients under-staged on pre-operative CT and have not undergone any type of induction therapy to ensure that the biology of the disease is better established.
Full table
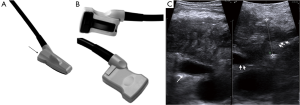
The example that will be presented below will demonstrate encasement of the celiac axis and a long superior mesenteric artery (SMA) involvement. The key to safe and effective IRE is to ensure that you have the highest quality ultrasonography (US) imaging system, with at least a high definition screen and would recommend harmonic imaging to gain the highest quality imaging as possible. I have found that the easiest and most accurate ultrasound technique is performed with minimal to no dissection prior to ultrasound and use the ultrasound to image on top of the stomach using a transgastric technique with either a thin finger/iprobe or a biplane probe (Figures 1 and 2). The reason for this is that the stomach provides the most consistent ultrasound crystal apposition and thus the best image quality and accuracy with the least amount of artifact. Intraoperative ultrasound imaging has become our gold standard for elucidating whether a patient has a true locally advanced tumor or a borderline resectable tumor.

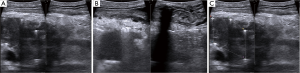
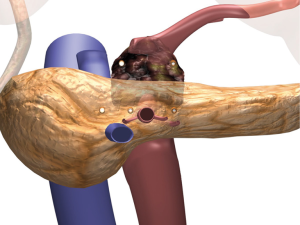
Once local advancement is confirmed and an in situ IRE is then planned, imaging of the tumor and the surrounding structures is then performed in order to obtain axial, anterior/posterior as well cranial/caudal maximum tumor diameters. Vital structures that need to be included in those diameters for appropriate needle placement are also assessed (Figure 1). Given that a majority of pancreatic neck tumors’ longest axis is axial with infiltration of the celiac axis median 3 cm, range 2 to 4 cm, it is not uncommon to have an anterior-posterior tumor maximum depth between 2.5 and 3.0 cm in size. Based on the maximum axial diameter appropriate needles are placed at exactly 2.0 cm apart so that the entire tumor and an approximate 1.0 cm margin of normal soft tissue is included in the IRE plane.
As demonstrated in Figure 3, this most commonly requires four or five needles in a trans-mesocolon approach, two to three needles posteriorly, the other underneath the superior mesenteric vein (SMV) but to the patients’ right of the SMA, and the second to the left of the SMA, and a third to the patients left toward the spleen in a row of three probes. One to two additional probes are then placed in a more anterior approach, most commonly 1.5 cm anteriorly such that a triangle or an oblong square is then obtained (Figures 3 and 4). We use spacers at 2.0 cm intervals build off that initial needle to ensure adequate margin posterior to the SMA and place the needles on either side of the SMA to ensure adequate treatment margin(s). Needles are then placed in order to obtain complete bracketing of the tumor.
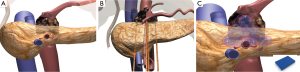
The optimal placement of the IRE needles is performed through continuous intraoperative ultrasound from the insertion of the needle into the tissue so that the needle tip is followed at all times during needle placement. Similar to treating a pancreatic head tumor, I have found that placing these needles through the transverse mesentery, with care not to damage the transverse colon vessels, is easier because it allows normal soft tissue to bracket the pancreatic head tumor as well as to allow for appropriate inferior margin to be obtained during pullbacks of the needle (10). Thus, the transverse mesocolon is grasped and raised anteriorly out of the abdomen by an assistant and then the surgeon’s dominant hand directs the needle into the tissue, while her/his non-dominant hand utilizes the ultrasound probe to ensure accurate and appropriate needle placement. It cannot be overemphasized that an atraumatic needle placement should be performed to ensure that the needle does not damage the underlying vital structures, namely the SMV, portal vein, SMA, and hepatic artery. Vascular needle trauma may induce underlying vascular thrombosis, especially given the potential hypercoagulable state in a patient with pancreas cancer. In some instances the tumor is extending superior above the celiac axis and requires an overlapping IRE to be performed with needles placed through the lesser sac superior to the lesser curve of the stomach.
Care should also be undertaken that the maximum needle exposure to perform safe IRE of the pancreas should be 1.0 to 1.5 cm because of the significant fibrotic nature of these tumors and a larger needle exposure will not be tolerated by the gland or the underlying soft tissue to be treated (16). We have previously published that a greater probe exposure leads to high current conditions and the potential for thermal damage if these high current conditions are allowed to persist. Thus the maximum probe exposure should be 1.5 cm or less (16).
Following appropriate needle placement and ultrasound confirmation of appropriate spacing, those spacing measurements are entered into the energy unit’s software, which allow for optimal voltage and pulse length delivery. Standard default voltage of 1,500 volts per cm is initiated with planned delivery of 90 pulses and a pulse width of 70 to 90 microseconds. As we have recently published 20 pulses are delivered initially and then the delivery is halted in order to assess the underlying amperage draw to establish optimal voltage and pulse widths (14). If the current amperage draw for these first 20 pulses is less than 35 amps I believe that this is an appropriate voltage per cm and pulse widths for safe and effective electroporation. Energy is delivered between all needle pairs (Figure 4C) and evaluation of the energy delivered is then assessed for each pair in order to demonstrate a change in current amperage draw, which has been found to be an appropriate surrogate marker of change and resistance. This change in resistance is of utmost importance to ensure against reversible electroporation, which would lead to ineffective therapy and electroporation failure (14). Once effective current delivery has been confirmed between all pairs the needles are pulled back the appropriate distance such that no overlapping treatments are performed (10). Sequential pullbacks are performed in order to obtain adequate margins both superiorly and inferiorly. Each probe pair is then treated again following subsequent pull back and again is re-treated for a total 180 pulses, or even in a rare instance 270 pulses if the current draw does not appropriately change over each 90 pulse delivery. Following optimal pulse delivery at each needle placement and providing appropriate margins are felt to be obtained with the needle placement, the needles are removed without the need for any additional hemostatic procedures (i.e., suture ligature, etc.) in most cases. Another probe configuration using a 5-probe formation is sometimes needed based on a width of the axial plane of the tumor that at times narrows anteriorly (Figure 5). Post IRE ultrasound can be performed to document vital vessel patency, but because of the underlying edema induced by IRE, ultrasound IRE efficacy is not possible (Figure 6). This edema post IRE is another reason why attempting to perform overlapping ablations is not recommend since the IRE edema/artifact can make it difficult to place needles safely in an overlapping configuration with the degree of accuracy that is required.

As reported previously with panc head tumors (10), the underlying tissue edema does require any specific surgical procedures to control needle site bleeding. At most, if needle placement has punctured one of the small transverse mesocolon vessels, a suture ligature is necessary. It should be noted that continuous intraoperative ultrasound is performed during all IRE delivery in order to assess energy delivery as well as to continually evaluate vascular patency if indeed the treating surgeon feels necessary.
An abdominal drain is usually not placed in patients who undergo just in situ IRE.
The postoperative management of these patients is fairly standard and follows guidelines for any type of pancreatic resection. The return of gastrointestinal (GI) function and the length of stay still remain approximately four to six days. An initial efficacy CT scan is not obtained until three months post IRE because of the protracted method of action that occurs with IRE. Imaging prior to that will be inaccurate because of the edema and ongoing apoptosis, which is the most common method of IRE induced cell death as demonstrated in large porcine model experiments (16,18). Commonly, re-initiation of systemic chemotherapy is performed before this three month CT scan. A patient in whom external beam radiation therapy is felt necessary (i.e., to cover regional lymph nodes) is also initiated prior to this three month CT scan if the multidisciplinary team feels necessary.
We have recently published both safety and efficacy data in locally advanced pancreatic cancer (8,9). Key to the success in the treatment of this challenging disease is patient selection and clear collaboration with medical oncology and radiation oncology.
Acknowledgements
Disclosure: The author is a consultant for Angiodynamics.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Bilimoria KY, Bentrem DJ, Merkow RP, et al. Application of the pancreatic adenocarcinoma staging system to pancreatic neuroendocrine tumors. J Am Coll Surg 2007;205:558-63. [PubMed]
- Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371-6. [PubMed]
- Al-Sakere B, André F, Bernat C, et al. Tumor ablation with irreversible electroporation. PLoS One 2007;2:e1135. [PubMed]
- Edd JF, Horowitz L, Davalos RV, et al. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng 2006;53:1409-15. [PubMed]
- Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223-31. [PubMed]
- Davalos RV, Otten DM, Mir LM, et al. Electrical impedance tomography for imaging tissue electroporation. IEEE Trans Biomed Eng 2004;51:761-7. [PubMed]
- Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg 2012;215:361-9. [PubMed]
- Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol 2013;20 Suppl 3:S443-9. [PubMed]
- Martin RC. Irreversible electroporation of locally advanced pancreatic head adenocarcinoma. J Gastrointest Surg 2013;17:1850-6. [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [PubMed]
- Dunki-Jacobs EM, Philips P, Martin RC 2nd. Evaluation of thermal injury to liver, pancreas and kidney during irreversible electroporation in an in vivo experimental model. Br J Surg 2014;101:1113-21. [PubMed]
- Dunki-Jacobs EM, Philips P, Martin RC 2nd. Evaluation of resistance as a measure of successful tumor ablation during irreversible electroporation of the pancreas. J Am Coll Surg 2014;218:179-87. [PubMed]
- Martin RC II. Biplane US imaging (axial imaging left panel, sagittal imaging right panel) demonstrating locally advanced pancreatic cancer involving the celiac bifurcation of the splenic and hepatic artery. Asvide 2015;2:009. Available online: http://www.asvide.com/articles/463
- Bower M, Sherwood L, Li Y, et al. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol 2011;104:22-8. [PubMed]
- Martin RC II. Biplane US imaging demonstrating US images post IRE, which only demonstrates edematous changes and thus does not allow for accurate post IRE confirmation utilizing the current B-mode US that is available in the United States. Asvide 2015;2:010. Available online: http://www.asvide.com/articles/464
- Charpentier KP, Wolf F, Noble L, et al. Irreversible electroporation of the pancreas in swine: a pilot study. HPB (Oxford) 2010;12:348-51. [PubMed]


