Surveillance for asymptomatic recurrence in resected stage III colon cancer: does it result in a more favorable outcome?
Introduction
Colorectal cancer (CRC) is the third most common malignancy and cause of cancer related mortality in North America. The death rate from CRC has been annually declining by 3% since 2000, largely attributed to early detection and/or treatment (1). Around 80% of patients do not have metastatic disease at the time of diagnosis and are potentially eligible for curative intent surgery (2). Subsequently, adjuvant chemotherapy (AC) improves survival in patients with early stage high-risk colon cancer (CC) (3-5). Unfortunately, approximately one-third of curatively treated patients suffer a recurrence, mostly within the first three years following surgery (6). Complete resection of CRC liver metastases can result in close to 40% 5-year survival (7), and similar results have been observed with complete resection of lung metastases (8).Consequently, many organizations have published surveillance recommendations, primarily in an attempt to identify recurrences at a resectable stage (9-11).
Several randomized clinical trials (RCT) have been published on intensive surveillance post CRC resection, spanning many decades, and examining various permutations of follow-up schedules and tests (12-20). Meta-analyses have suggested an overall survival (OS) benefit to intensive follow-up, perhaps mediated through earlier (approximately 6 months) recognition of metastatic disease amenable to potentially curative metastasectomy (21-23). However, many of these RCTs were performed with moderate methodological quality (21). Furthermore, disease-specific survival, when reported, was not improved (15,17), thereby questioning whether the observed OS is attributable to CC directed therapy or perhaps other factors (23). Indeed, it has been estimated that only about 20% of the observed OS benefit could be attributed to resection of metastatic disease, and the balance of benefit may come from increased psychological support and well being, changes in dietary and lifestyle factors, or improved treatment of coincidental co-morbidities (24). Lastly, many of these RCTs were performed before or during landmark changes in AC (25), introduction of new agents in advanced disease, and uptake of hepatic resection (26). The results of these RCTs may therefore not be generalizable to the contemporary oncology clinic.
The recently published FACS study had planned to investigate OS of intensive surveillance with CEA or CT, alone or in combination, compared to minimal follow-up. Unfortunately, due to recruitment issues, the primary end point was amended to surgical treatment of recurrence with curative intent. FACS showed that between 12 and 20 patients needed to undergo intensive surveillance to detect 1 potentially resectable recurrence with curative intent, but there was no difference in OS (20). The ongoing COLOFOL and GILDA trials are investigating different surveillance schedules without a control no surveillance arm (27,28).
Given the uncertain OS benefit of intensive surveillance and outdated clinical trials, the aim of this study was to evaluate outcomes of intensive surveillance in a modern population-based cohort study. Furthermore, we focused the analysis on stage III CC patients, who have the highest risk of recurrence.
Methods
Patients
A retrospective chart review was conducted on all patients with resected stage III CC patients who initiated AC with either 5-fluorouracil (5-FU) or capecitabine plus oxaliplatin between 2006 and 2011 at the British Columbia Cancer Agency (BCCA). Institutional REB approval was obtained before beginning the review.
Surveillance recommendations at the British Columbia Cancer Agency (BCCA)
Over the study period, the BCCA had a standardized surveillance strategy for all resected stage III CC patients based on guidelines from the NCCN, ESMO and ASCO with the following recommendations: history and physical examination with CEA measured every3 months for the first 2 to 3 years, and every 6 months thereafter until year 5; abdominal imaging in the form of a computed tomography (CT) of the abdomen and pelvis or ultrasound every 6 months for the first 2 to 3 years, and annually thereafter until year 5. A yearly chest X-ray was recommended for patients with rectal cancer. Colonoscopy was to be performed within the first year of diagnosis and further endoscopic recommendations were made by the endoscopist. Patients were referred back to primary care physicians for surveillance follow-up at the discretion of the treating oncologist at the BCCA.
Data collection
Data was abstracted from the electronic medical record by two authors (Martin Smoragiewicz and Renata D’Alpino Peixoto). Data collection included baseline patient characteristics, tumors characteristics from pathology and operative reports, whether patients underwent potentially curative metastasectomy, and post-recurrence chemotherapy treatment. The method of recurrence detection was determined by which modality first prompted investigations leading to a diagnosis of a recurrence: surveillance CT, surveillance rising CEA, or symptoms prompting additional investigations. Recurrences were classified as locoregional only (recurrence in the mesentery, retroperitoneum, peritoneum, or at the surgical anastomosis), liver and/or lung only, and other metastatic.
OS1 and OS2 were measured from the date of recurrence or date of initial surgery, respectively, to the date of death or last follow-up. Relapse-free survival (RFS) was determined from the date of initial CC surgery to the date of recurrence.
Statistical analyses
Baseline characteristics of patients with surveillance and symptom-detected recurrences were compared using Pearson chi-square test. The OS and RFS survival curves were estimated using the Kaplan-Meier method and compared using the log-rank Mantel-Cox test. All reported P values are two sided and 95% confidence intervals are included. Statistical significance was defined as a P value <0.05. Statistical analyses were performed using SPSS.
Results
The BCCA treated 635 stage III CC patients with oxaliplatin based AC between 2006 and 2011. A total of 175 patients (27.5%) recurred after a median follow-up of 67.7 months. Most recurrences (149 patients, 85%) were identified by surveillance CT (44%) or a rising CEA (41%). There were no differences in baseline characteristics between patients with recurrences detected by surveillance or symptoms, except for the latter group being slightly older (median age 61 vs. 66, P=0.03) and containing more high-grade tumors (19% vs. 7%, P=0.01). However, the surveillance-detected recurrences were more likely to be liver and/or lung limited. Furthermore, patients with surveillance-detected recurrences were more likely to undergo potentially curative metastasectomy (39% vs. 7%, P=0.002) and receive any chemotherapy (70% vs. 50%, P=0.03) (Table 1).
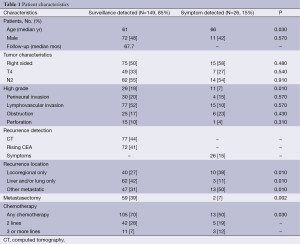
Full table
Compared with symptom-detected recurrences, patients with surveillance-detected recurrences had a shorter median RFS (18.5 vs. 25.3 months, P<0.001, HR 1.82; 95% CI: 1.16-2.85, Figure 1A), and longer median OS post recurrence detection (OS1, 28.5 vs. 6.5 months, P<0.001, HR 0.37; 95% CI: 0.23-0.60, Figure 1B). However, the median OS from the time of initial surgery was not significantly different between these groups (OS2, 50.9 vs. 39.1, P=0.091, HR 0.66; 95% CI: 0.41-1.07, Figure 1C).

Discussion
It is difficult to ascertain from our data whether patients with symptom-detected recurrences represent interval cases while on intensive surveillance, or are patients that did not adhere to surveillance recommendations. Indeed, though the BCCA treats most cancer patients in the province of British Columbia and this cohort is therefore a representative modern population-based sample of stage III CC, many patients are referred back to their primary care physicians for surveillance with difficult access to records. The median 6.8-month delay in recurrence diagnosis and age difference (29) between the symptom and surveillance-detected groups would support the latter case. However, in the FACS study, about 17% of recurrences in the CEA/CT surveillance arm were interval cases (20), which is comparable to the proportion of symptom-detected recurrences in our study. Due to this issue, it is difficult to compare our results with studies including patients assigned to minimal or no surveillance.
Regardless of this point, it is concerning for intensive surveillance policies that patients with asymptomatic recurrences did not have an OS benefit compared with patients with symptomatically detected recurrences. This is particularly puzzling given that the former group received more potentially curative metastasectomy and chemotherapy. Furthermore, the potential length time bias from some symptom-detected recurrences being interval cases should have disadvantaged this group in survival analyses. Nonetheless, around 50% of the symptom-detected group did receive at least 1 line of chemotherapy, and a couple of patients underwent potentially curable metastasectomy, thereby perhaps mitigating any OS benefit from asymptomatic detection. It is difficult to compare our results given many of the surveillance RCTs do not report post-recurrence chemotherapy treatment rates (12-20). It is clear, however, that some symptomatic recurrences remain resectable as observed in this study and reported elsewhere in the literature (30). Of course, there was an 11.8-month OS trend (P=0.091) in favor of the former group that may have become significant with increasing sample size. This further supports the point, also noted in the FACS trial (20), that if there is a survival benefit to any surveillance strategy in unselected patients, it is likely small.
The results of this study should not dissuade physicians from enrolling stage III CC patients onto intensive surveillance protocols. Complete resection of liver and/or lung metastases in appropriately selected patients is associated with favorable long-term survival (7,8,31), and this study showed patients with asymptomatic recurrences are more likely to undergo potentially curative metastasectomy. Furthermore, given chemotherapy treatment of asymptomatic metastatic disease improves survival (32), a higher probability of receiving chemotherapy offers an opportunity to increase OS with improving chemotherapy treatment options. Rather, our results should serve to justify further study into identifying subsets of patients that may benefit most from intensive surveillance, and defining the optimal surveillance sequence. The ongoing GILDA and COLOFOL trials should shed some light on the latter question (27,28).
We restricted our analysis to stage III CC patients, who have the highest risk of recurrence. Interestingly, the FACS study showed approximately 5% of patients with stages I, II and III underwent potentially curative resection, suggesting intensive surveillance offers a similar benefit across stages of disease. However, given the overall lower risk of recurrence in earlier stages of disease, potentially curative resections play a proportionately greater role in stage I and II CC. Future population based research should evaluate the impact of surveillance on earlier stages of disease.
The strength of our study is that it offers insight into real world practice with a modern population-based cohort of moderate size. Furthermore, to our knowledge, this is the only study to only include stage III CC patients, who have the highest risk of recurrence. Limitations include the retrospective nature and lack of detailed data on surveillance follow-up. However, many of our findings are in agreement with the known literature.
Conclusions
The OS impact of detecting an asymptomatic recurrence in stage III CC is unclear. However, patients with asymptomatic recurrences are more likely to receive potentially curative metastasectomy and chemotherapy.
Acknowledgements
Authors’ contributions: RD Peixoto designed the overall study with contributions from M Smoragiewicz and H Lim. RD Peixoto and M Smoragiewicz collected and analyzed data, and co-wrote the paper. RD Peixoto, H Lim and M Smoragiewicz discussed and edited the paper.
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. eds. AJCC cancer staging manual. 7th Edition. New York, NY: Springer, 2010:237-46.
- Smith RE, Colangelo L, Wieand HS, et al. Randomized trial of adjuvant therapy in colon carcinoma: 10-year results of NSABP protocol C-01. J Natl Cancer Inst 2004;96:1128-32. [PubMed]
- Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol 1993;11:1879-87. [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [PubMed]
- O'Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol 2008;26:2336-41. [PubMed]
- Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012;4:283-301. [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis.Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Fort Washington, PA: NCCN, 2014: Ver. 3.2014. Available online: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- Meyerhardt JA, Mangu PB, Flynn PJ, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2013;31:4465-70. [PubMed]
- Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. [PubMed]
- Wattchow DA, Weller DP, Esterman A, et al. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br J Cancer 2006;94:1116-21. [PubMed]
- Secco GB, Fardelli R, Gianquinto D, et al. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg Oncol 2002;28:418-23. [PubMed]
- Schoemaker D, Black R, Giles L, et al. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology 1998;114:7-14. [PubMed]
- Kjeldsen BJ, Kronborg O, Fenger C, et al. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg 1997;84:666-9. [PubMed]
- Mäkelä JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Arch Surg 1995;130:1062-7. [PubMed]
- Ohlsson B, Breland U, Ekberg H, et al. Follow-up after curative surgery for colorectal carcinoma. Randomized comparison with no follow-up. Dis Colon Rectum 1995;38:619-26. [PubMed]
- Pietra N, Sarli L, Costi R, et al. Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis Colon Rectum 1998;41:1127-33. [PubMed]
- Rodríguez-Moranta F, Saló J, Arcusa A, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol 2006;24:386-93. [PubMed]
- Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014;311:263-70. [PubMed]
- Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2007;CD002200. [PubMed]
- Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 2002;324:813. [PubMed]
- Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum 2007;50:1783-99. [PubMed]
- Renehan AG, Egger M, Saunders MP, et al. Mechanisms of improved survival from intensive followup in colorectal cancer: a hypothesis. Br J Cancer 2005;92:430-3. [PubMed]
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. [PubMed]
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677-83. [PubMed]
- Wille-Jørgensen P, Laurberg S, Påhlman L, et al. An interim analysis of recruitment to the COLOFOL trial. Colorectal Dis 2009;11:756-8. [PubMed]
- Grossmann EM, Johnson FE, Virgo KS, et al. Follow-up of colorectal cancer patients after resection with curative intent-the GILDA trial. Surg Oncol 2004;13:119-24. [PubMed]
- Hu CY, Delclos GL, Chan W, et al. Post-treatment surveillance in a large cohort of patients with colon cancer. Am J Manag Care 2011;17:329-36. [PubMed]
- Graham RA, Wang S, Catalano PJ, et al. Postsurgical surveillance of colon cancer: preliminary cost analysis of physician examination, carcinoembryonic antigen testing, chest X-ray, and colonoscopy. Ann Surg 1998;228:59-63. [PubMed]
- Bernard BD, Gresham G, Chen L, et al. A comparison of survival by site of metastatic resection (MR) in metastatic colorectal cancer (mCRC). J Clin Oncol 2014;32:abstr 3529.
- Nordic Gastrointestinal Tumor Adjuvant Therapy Group. Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. J Clin Oncol 1992;10:904-11. [PubMed]


