Adjuvant radiation therapy for pancreatic cancer: a review of the old and the new
Introduction
Despite improvements in surgical management, chemotherapy, and chemoradiation therapy (CRT) approaches, pancreatic cancer (PC) continues to be a formidable disease for oncologists. Localized PC is categorized on a spectrum spanning from resectable to locally advanced based primarily on the presence or absence of vascular involvement. The determination of resectability involves prospective assessment employing imaging studies, predominantly CT scan, but also MRI and endoscopic ultrasound. Resectable disease is defined by the absence of distant metastases and lack of involvement of the adjacent vasculature [i.e., celiac axis, hepatic artery, superior mesenteric artery (SMA), superior mesenteric vein (SMV) or portal vein (PV)] (1). Though a subjective category with variability between surgeons and institutions, borderline resectable disease allows for venous involvement (PV or SMV) that is deemed resectable and where reconstruction is feasible, as well as lesions with limited SMA abutment (<180°) (2,3).
Surgery represents the only potentially curative treatment for patients with PC. Approximately 20% of patients will present with resectable disease. Despite the ability to remove all gross disease, outcomes for this group of patients are limited by high rates of local (50-90%) as well as distant (peritoneal: 20-35%; liver 20-90%) recurrence (4-7). Local recurrence is a significant driver of morbidity (i.e., pain, ulceration, bleeding, obstruction, cholangitis). Furthermore, uncontrolled local disease is often associated with distant failure as well as subsequent mortality (8). Adjuvant therapies (ATs) including CRT have been extensively investigated with hopes of reducing rates of recurrence and improving long-term outcomes. This review will first discuss the large randomized trials of adjuvant chemotherapy and CRT and then focus on the contemporary role of adjuvant RT. Particular attention will be paid to the emerging role of novel radiation techniques.
Adjuvant therapy (AT) for resected pancreatic cancer (PC)
In an attempt to improve outcomes for this group of patients, a number of studies have been conducted exploring the efficacy of ATs (Table 1). Many of the early studies investigating AT for resected PC are limited in their interpretation and generalizability by flaws in study design and analyses. For example, many failed to include pre-operative imaging into the initial determination of resectability (9,10,12,14,16). Most did not include central pathology review (9,12,14,16) or post-operative imaging for re-staging prior to initiation of ATs (9,10,16). Nonetheless, these trials inform current treatment strategies and have guided ongoing and future investigations.
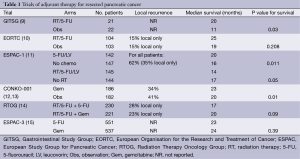
Full table
Historical trials of adjuvant therapy (AT)
The GITSG 9173 study established the role for adjuvant CRT. This trial enrolled 43 of an intended 100 patients with PC having undergone pancreaticoduodenectomy (PD) and randomized to no further therapy or adjuvant, split course CRT with 5-fluorouracil (5-FU) (9). Treatment in the CRT arm consisted of a course of radiation to 40 Gy with a planned 2-week treatment break after the initial 20 Gy. Bolus 5-FU was administered weekly during RT and for up to two years thereafter. Though the trial was closed early due to poor accrual, an OS benefit was found with a median survival of 20 vs. 11 months and 2-year survival rates of 42% vs. 15% (P=0.03). The GITSG trial established adjuvant CRT as an acceptable adjuvant treatment for resected PC.
An attempt to replicate these results was conducted by the European Organisation for Research and Treatment of Cancer (EORTC). The trial enrolled 218 patients and randomized similarly between observation and split course CRT with 5-FU (10). Similar to the GITSG study, RT was delivered in a split course to 40 Gy. The 5-FU was delivered as a continuous infusion. Unlike in the GITSG study, there was no significant survival benefit with AT. With long-term follow-up, 5-year survival rates were 25% (CRT) vs. 22% (surgery alone) (17). A notable difference of the EORTC study was inclusion of 104 peri-ampullary tumors. A subset analysis was performed including only pancreatic head tumors, demonstrating a trend towards improved 2-year overall survival with AT with a median 17 (CRT) vs. 13 months (surgery alone) (17).
The European Study Group for Pancreatic Cancer-1 (ESPAC-1) study was a 2×2 study designed to investigate both adjuvant chemotherapy and adjuvant CRT compared to observation following resection. Patients were randomized to observation, chemotherapy alone, CRT, or CRT followed by maintenance chemotherapy (16). Clinicians were encouraged to enroll in the 2×2 randomization but given the option to select their patients’ randomization. Chemoradiation was delivered in a split course fashion, consistent with the GITSG and EORTC trials. Chemotherapy consisted of bolus 5-FU and folinic acid administered days 1-5 and repeated every 28 days for 6 cycles. Of the 541 patients enrolled, 285 were randomized in the 2×2 design. Long-term results were reported with a median 47 months follow-up and when restricted to patients in the 2×2 randomization, CRT was found to result in a survival detriment (median survival 14 vs. 17 months) whereas a survival benefit was found for adjuvant chemotherapy (median survival 20 vs. 16 months) (11). In this study, recurrence rates were high regardless of treatment arm. Similar to the aforementioned trials, median survival was poor and the ESPAC-1 trial stands alone in showing a survival detriment with CRT.
These early investigations of adjuvant CRT are limited in their interpretation and generalizability by flaws in trial design and conduct. These trials utilized split course, low dose RT schedules with no RT quality assurance and bolus 5-FU. A dose of 40 Gy is likely inadequate to establish disease control while split course radiation prolongs overall treatment time, reducing potential biological effectiveness. Post-operative complications precluding adjuvant treatment occurred in nearly 20-30% of patients. In reality, the GITSG study tested two interventions against the control by incorporating both adjuvant CRT and additional adjuvant chemotherapy. Furthermore, the study was hindered by poor enrollment and significant protocol violations. The EORTC trial included a heterogeneous population of peri-ampullary and pancreatic tumors, potentially diluting the benefit of CRT among PC patients. Results of the ESPAC-1 study have been questioned, among many reasons, due to its 2×2 design and concerns for selection bias. The results of these early trials, though flawed, guided treatment for patients with resected PC and informed the future trials.
Modern trials of adjuvant therapy (AT)
Given the lack of benefit of CRT seen in the EORTC and ESPAC-1 studies, further investigation in Europe has attempted to optimize adjuvant chemotherapy strategies. The German Charité Onkolgie (CONKO-001) trial (12) investigated the efficacy of adjuvant gemcitabine whereas the ESPAC-3 trial compared adjuvant 5-FU vs. gemcitabine (18). In the United States, the Radiation Therapy Oncology Group (RTOG) conducted a randomized trial comparing adjuvant 5-FU-based CRT with either additional 5-FU or gemcitabine (14).
The German CONKO-001 trial enrolled 354 patients post-PD with R0 (83%) or R1 resection and randomized to observation or gemcitabine (12). Gemcitabine was administered in three weekly infusions for a total of six cycles. With a median follow-up among survivors of 4.5 years, gemcitabine resulted in a near doubling of disease-free survival (DFS), with median intervals of 13 vs. 7 months for observation. Grade 3-4 toxicities were primarily hematologic. With longer follow-up adjuvant gemcitabine resulted in reduced risk of death (HR 0.76, P=0.01) (13).
The ESPAC-3 trial similarly enrolled 1088 patients having undergone PD with R0 (65%) or R1 resection and randomized to observation, adjuvant fluorouracil (bolus ×6 cycles) or gemcitabine (×6 cycles) (15). Following the publication of ESPAC-1, the observation arm was closed and the trial became a comparison of 5-FU and gemcitabine. With a median follow-up of 34 months, there was no difference in survival seen between adjuvant gemcitabine or 5-FU with median survivals of 24 and 23 months, respectively. Rates of grade 3-4 toxicities were higher with 5-FU (primarily diarrhea, stomatitis) compared to gemcitabine (hematologic).
After improved results of gemcitabine in patients with metastatic disease (19), the RTOG conducted a randomized trial (97-04) investigating whether gemcitabine compared with continuous infusion 5-FU, administered before and after standard 5-FU based CRT (50.4 Gy), could improve outcomes in the adjuvant setting (14). The study enrolled patients having undergone PD with R0 or R1 resections. Chemotherapy was administered for three weeks prior and 12 weeks following chemoradiation and all RT plans underwent prospective quality assurance. With a median follow-up of 4.7 years among surviving patients, the addition of gemcitabine led to a trend in improved survival (mean 17 vs. 20 months, P=0.09), although at the expense of higher grade 4 hematologic toxicity. Results among the 86% of patients with pancreatic head tumors suggested a benefit for gemcitabine (14), though with longer follow-up there was no statistically significant difference (20). Patients with a post-operative CA 19-9 level ≤90 experienced a significantly longer median survival compared to >90, at 23 vs. 10.4 months respectively (21). This finding was confirmed on multivariate analysis (HR 3.34, P<0.0001). One hypothesis is that this group of patients with higher CA-19-9 levels may harbor micrometastatic disease, which may have implications for selection of appropriate adjuvant treatments. A secondary analysis assessed outcomes for patient treated with per-protocol RT (n=216) as compared to those with protocol violations (n=200) (22). It was found that patients treatment with per-radiotherapy protocol had significantly improved overall survival. Moreover, on multivariate analysis, per-protocol treatment was more closely linked with survival than was the randomized treatment assignment.
What are the summative conclusions of the randomized trials of AT reported to date? Based on the results of the CONKO-1 and the ESPAC trials, adjuvant chemotherapy has been shown to consistently improve outcomes. Gemcitabine appears superior to 5-FU in terms of toxicity. The results of these trials are less clear on the role of adjuvant CRT. The GITSG, EORTC, and ESPAC-1 trials resulted in differing conclusions, though this may be at least partially explained by the many deficiencies of these studies as previously discussed. The more recent RTOG study is the only trial to incorporate “modern” RT and quality assurance of RT plans, yet the trial was not designed to test the efficacy of CRT.
Available data does suggest lower rates of local recurrence with the incorporation of optimal CRT. In RTOG 97-04, the local recurrence rate was only 26% despite substantial proportions of patients with T3/T4 disease (75%), involved lymph nodes (66%) and positive margins (34%). The EORTC and ESPAC-1 trials, with suboptimal CRT techniques and omission of RT in some ESPAC-1 patients, resulted in substantially higher local recurrence rates (36-62%) despite including predominantly patients with T1/T2 disease (EORTC), negative margins (EORTC and ESPAC-1) and low CA 19-9 levels (CONKO-001). Similarly, local recurrence rates in the (CONKO-001) (34-41%) and ESPAC-3 (63%) trials compare unfavorably to the RTOG and other trials incorporating adjuvant CRT. The ability of adjuvant CRT to reduce local recurrence rates was demonstrated by a smaller randomized phase II study conducted in patients undergoing R0 resection (23). In this study 90 patients were randomized between four cycles of gemcitabine or two cycles of gemcitabine followed by CRT with concurrent gemcitabine. While there was no difference in DFS or OS, there was a reduction in local recurrence as first progression with chemoradiation (11% vs. 24%). As more efficacious systemic therapies are developed, the ability to safely achieve local control may become increasingly important.
The ongoing RTOG 08-48 is a phase III trial randomizing patients post-PD to five cycles of gemcitabine or gemcitabine and the tyrosine kinase inhibitor, erlotinib. The rationale of erlotinib was based on efficacy data in the locally advanced or metastatic setting, though this arm has now been closed (24,25). Patients are then re-imaged to evaluate for progression, and if no progression, are randomized to one additional cycle of chemotherapy or one additional cycle of chemotherapy (six cycles total) followed by 5-FU-based CRT. The study utilizes modern radiation techniques to a dose of 50.4 Gy and incorporates centralized, prospective quality assurance of RT plans. In Europe, the ESPAC-4 trial seeks to investigate the efficacy of adding capecitabine to standard gemcitabine in the adjuvant setting. The results of these trials will potentially provide valuable information regarding the optimal adjuvant treatment strategy as well as further assess the role of CRT.
Given the conflicting results of randomized trials, several groups have published their institutional results of treatment with adjuvant CRT. A prospective series from Johns Hopkins reports outcomes of 616 patients undergoing PD for pancreas cancer, of which 271 received adjuvant 5-FU based CRT (26). Pathologic tumor characteristics between those who did and did not receive CRT were similar in regards to involved nodes (82% vs. 79%, NS) and positive margins (48% vs. 42%, NS). With a median follow-up of 18 months, patients receiving AT showed statistically and meaningfully improved median survival time (21 vs. 14 months) as well as 5-year overall survival (20% vs. 15%). This benefit persisted after adjusting for covariates and an analysis of treatment effect showed the benefit to exist for both positive and negative margins. A second series from the Mayo Clinic reported on 466 patients with T1-3N0-1 PC undergoing curative, margin negative resection, 274 of who received adjuvant CRT (27). Despite more patients with T3 tumors, involved nodes, and high-grade disease, survival was superior for patients receiving CRT (median 25 vs. 19 months; 2-year OS 50% vs. 39%). Analyses of the effect of CRT by tumor characteristics confirmed a survival benefit for patients with involved lymph nodes and high-grade tumors, but not for patient with uninvolved nodes. A follow-up matched pair analysis, combining data from both institutions (496 patients), confirmed a survival benefit for adjuvant chemoradiation with a relative risk of 0.59 (0.48-0.72) (28).
Novel radiation therapy (RT) techniques
In the decades since the inception of the GITSG study, significant advances in radiation technology have allowed for more conformal delivery of dose to target volumes. Intensity modulated RT (IMRT) and stereotactic body RT (SBRT) are two such techniques. Unlike 3-dimensional conformal RT, IMRT incorporates a planning technique, called inverse planning, whereby both target volumes and organs at risk are delineated by the radiation oncologist. A treatment plan is then generated through an optimization process that uses volumetric and dosimetric constraints (i.e., radiation prescription) for both target volumes and organs at risk, as inputs. IMRT breaks up a typical radiation treatment field into smaller “beamlets”. It is implemented either as dynamic IMRT (collimating leaves move in and out of the radiation beam path during treatment) or as “step and shoot” IMRT (leaves change field shape while the machine is off). The cumulative effect is that the prescription dose conforms around delineated target volumes, significantly reducing doses to adjacent normal tissues.
Stereotactic body RT [also known as stereotactic ablative radiotherapy (SABR) and high-dose image guided radiotherapy (HIGRT)] can employ many of the same strategies and couples a high degree of anatomic targeting accuracy and reproducibility with high doses of ionizing radiation. This maximizes the cell-killing effect on the target while minimizing injury to adjacent normal tissues. Both SBRT and IMRT incorporate rigorous image guidance, accounting for day-to-day variations in location of the target volumes and adjacent normal tissues. The proposed benefits of a shortened course of RT are two-fold. First, radiobiological principles suggest that large fractional doses of radiation increase the biologically effective dose. Second, by shortening the overall treatment time, patients can more quickly proceed to systemic therapies.
A fundamental principle of these conformal radiation techniques is accurate delineation of target volumes. This requires an intimate knowledge of normal anatomy and patterns of lymphatic drainage. Appropriate delineation of target volumes must also thoroughly consider preoperative tumor features (determined by preoperative imaging) as well as account for surgical and pathologic features. In an effort to standardize this process, the RTOG has developed contouring guidelines which have been incorporated into the protocol of RTOG 0848 (29). The recommended contours are based on a combination of preoperative tumor location, surgical anastomoses, and nodal regions based on vasculature. A combined effort from Johns Hopkins and the University of Maryland investigators draws from their patterns of failure analysis of 202 patients with resected disease to generate target volumes (30). It was found that a target volume that would encompass 80% of recurrences could be generated by expanding a combined contour of the proximal CA and SMA by 2.0 cm right lateral, 1.0 cm left lateral, 1.0 cm anterior, 1.0 cm posterior, 1.0 cm superior, and 2.0 cm inferior. A volume encompassing 90% of recurrences could be generated by expanding an additional 1.0 cm right lateral, 1.0 cm left lateral, and 0.5 cm anterior. An example of IMRT is shown in Figure 1.
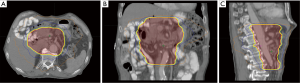
Clinical experiences utilizing IMRT
In the context of PC, IMRT has been employed in the locally advanced (31-35) and adjuvant settings (32,35,36) (Table 2). Given the small patient numbers in these series, they should be considered primarily as feasibility studies and for their toxicity assessments.
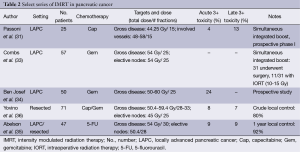
Full table
The University of Chicago published initial experience of IMRT with concurrent 5-FU in a mixed population of patients with resected disease (n=8), unresectable disease (n=13), and unresected recurrence (n=3) (32). In their study, radiation volumes included the tumor bed (45-50.4 Gy) or gross disease (50.4-59.4 Gy) and regional lymphatics (41.4-50.4 Gy). In six patients, dosimetric analysis of the IMRT and a 3-dimensional conformal plan was performed. They found statistical reductions in dose to the kidneys, small bowel, and liver. Treatment was relatively well tolerated and with a median follow-up of 14 months, a total of six acute and one late grade 3 or 4 non-hematologic toxicities occurred. With the caveat of small patient numbers, none of the eight patients who were resected experienced a local recurrence with a median follow-up of 17 months.
Investigators at the University of Michigan conducted a phase I/II prospective study of dose escalated (up to 60 Gy) IMRT with concurrent gemcitabine (34). In their series of 50 patients, radiation was delivered to gross disease only with customized margins allowing for target respiratory motion. Concurrent gemcitabine was delivered at full dose (1,000 mg/m2) to maximize local and distant control. Of note, prior studies had found full dose gemcitabine with concurrent RT to be unacceptably toxic (37). The current study hypothesized that the use of IMRT would improve the safety of this approach by reducing the dose to normal tissues. A total of 11 dose limiting toxicities occurred (52.5-57.5 Gy) including anorexia, nausea, vomiting or dehydration (n=7), duodenal bleed (n=3), and duodenal perforation (n=1). Two deaths were considered to be potentially due to therapy (peritonitis and duodenal perforation). The authors concluded that 55 Gy was a safe dose. Importantly, it was found that freedom from local progression (a secondary endpoint) was improved with dose escalation.
A combined series of 71 patients from the Johns Hopkins Medical Institutions and the University of Maryland is the largest to assess outcomes for IMRT employed in the setting of resected disease (36). Targets included elective coverage of the regional nodes (45 Gy) with a boost target encompassing the tumor bed (50.4-59.4 Gy). With a median follow-up of 2 years, 14 (20%) of patients experienced a local recurrence. Importantly, 9/14 local recurrences were without a distant component. Treatment was well tolerated with 8% grade 3 acute toxicity (no grade 4) and 7% late toxicity (small bowel obstruction or fistula).
Early clinical experience of SBRT and ongoing clinical trials
There is a paucity of available data detailing the efficacy and safety of adjuvant SBRT for PC. One of the few published reports comes from the University of Pittsburgh. In this series, 24 patients were treated with post-operative radiation with single fraction SBRT (20-24 Gy). With a median of 12.5 months of follow-up, grade 1-2 toxicity was 12.5%. No grade 3 or higher toxicities were reported and 19/24 patients were able to proceed to systemic gemcitabine-based chemotherapy. Freedom from local progression was 66%. Among 16 patients with positive resection margins, 10 (62.5%) were free of local progression (38).
There are at least two ongoing prospective studies of adjuvant SBRT. Building upon their early experience, the University of Pittsburgh is enrolling patients with resected disease and close or positive margins (NCT01357525). Radiation doses of 36 Gy in 12 Gy fractions are planned. The primary endpoint is local progression-free survival with a secondary analysis of quality of life. Investigators at Johns Hopkins are expanding on their experience using SBRT in a randomized phase II trial that investigates the safety and efficacy of an immune-modulating vaccine in conjunction with FOLFIRINOX (oxaliplatin, irinotecan, 5-FU, leucovorin). All patients will be treated with SBRT fraction sizes of 6.6 Gy for 5 days followed by FOLFIRINOX. The experimental arm will include the vaccine (NCT01595321). Results of these trials will provide important information regarding the safety of SBRT in the adjuvant setting.
Conclusions
Adjuvant chemotherapy has consistently led to improvements in outcomes for patients with PC following resection and should be incorporated into adjuvant treatment strategies. The role of adjuvant RT remains controversial. Early trials were flawed in their utilization of what is now recognized as sub-optimal RT leading to mixed results. Ongoing trials of adjuvant RT, such as RTOG 08-48, incorporate evidence-based delineation of target volumes and rigorous quality assurance. Results of this study will serve to clarify the role of adjuvant radiotherapy in resected PC patients. The incorporation of modern radiation techniques such as IMRT and SBRT hold the promise of maximizing dose to target volumes while minimizing dose to normal tissues, thus broadening the therapeutic window and improving disease outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2014;12:1083-93. [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [PubMed]
- Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [PubMed]
- Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer 1976;37:1519-24. [PubMed]
- Gudjonsson B. Cancer of the pancreas. 50 years of surgery. Cancer 1987;60:2284-303. [PubMed]
- Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer 1990;66:56-61. [PubMed]
- Whittington R, Bryer MP, Haller DG, et al. Adjuvant therapy of resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 1991;21:1137-43. [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [PubMed]
- Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [PubMed]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [PubMed]
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-85. [PubMed]
- Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg 2007;246:734-40. [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147-56. [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [PubMed]
- Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol 2011;18:1319-26. [PubMed]
- Berger AC, Garcia M Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 2008;26:5918-22. [PubMed]
- Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704--a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 2012;82:809-16. [PubMed]
- Van Laethem JL, Hammel P, Mornex F, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol 2010;28:4450-6. [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [PubMed]
- Hammel P, Huguet F, Van Laethem JL, et al. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study. J Clin Oncol 2013;31:abstr LBA4003.
- Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. [PubMed]
- Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol 2008;26:3511-6. [PubMed]
- Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol 2010;17:981-90. [PubMed]
- Goodman KA, Regine WF, Dawson LA, et al. Radiation Therapy Oncology Group consensus panel guidelines for the delineation of the clinical target volume in the postoperative treatment of pancreatic head cancer. Int J Radiat Oncol Biol Phys 2012;83:901-8. [PubMed]
- Dholakia AS, Kumar R, Raman SP, et al. Mapping patterns of local recurrence after pancreaticoduodenectomy for pancreatic adenocarcinoma: a new approach to adjuvant radiation field design. Int J Radiat Oncol Biol Phys 2013;87:1007-15. [PubMed]
- Passoni P, Reni M, Cattaneo GM, et al. Hypofractionated image-guided IMRT in advanced pancreatic cancer with simultaneous integrated boost to infiltrated vessels concomitant with capecitabine: a phase I study. Int J Radiat Oncol Biol Phys 2013;87:1000-6. [PubMed]
- Milano MT, Chmura SJ, Garofalo MC, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 2004;59:445-53. [PubMed]
- Combs SE, Habermehl D, Kessel K, et al. Intensity modulated radiotherapy as neoadjuvant chemoradiation for the treatment of patients with locally advanced pancreatic cancer. Outcome analysis and comparison with a 3D-treated patient cohort. Strahlenther Onkol 2013;189:738-44. [PubMed]
- Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2012;84:1166-71. [PubMed]
- Abelson JA, Murphy JD, Minn AY, et al. Intensity-modulated radiotherapy for pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 2012;82:e595-601. [PubMed]
- Yovino S, Maidment BW 3rd, Herman JM, et al. Analysis of local control in patients receiving IMRT for resected pancreatic cancers. Int J Radiat Oncol Biol Phys 2012;83:916-20. [PubMed]
- McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2001;19:4202-8. [PubMed]
- Rwigema JC, Heron DE, Parikh SD, et al. Adjuvant stereotactic body radiotherapy for resected pancreatic adenocarcinoma with close or positive margins. J Gastrointest Cancer 2012;43:70-6. [PubMed]


