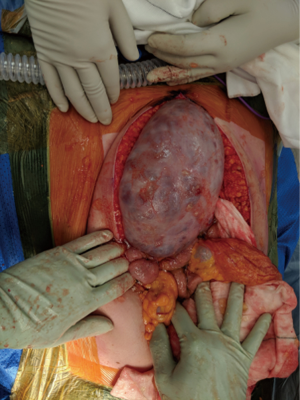
Surgical management of recurrent colon cancer
Introduction
Colorectal cancer is a common cancer. It is consistently either the second or third most common cancer across most develop countries (1-3). Not only is colorectal cancer common, it is also an important cause of cancer related deaths (4). In Australia, over 16,000 new cases were diagnosed in 2019, a 5% increase relative to 15,604 cases in 2015 (3). In general, men have a higher risk of developing colorectal cancer compared to women, and this is particularly true of rectal cancer, where there is a substantially higher risk for men compared to women.
The distribution of cancers of the colon and rectum is such that 70% of the cancers tend to occur in the colon while the remaining 30% occur in the rectum (5). This delineation between colon versus rectum is important as it has therapeutic, functional as well as prognostic implications (6). With the exception of stage IV colon cancer where treatment needs to be individualised depending on the site of metastases and whether or not the metastases are resectable, the treatment of stage I to III colon cancer is invariably surgery with or without adjuvant chemotherapy depending on final histopathology (7). There is limited role for radiotherapy, both in the neoadjuvant or adjuvant setting (8). The use of neoadjuvant chemotherapy in patients with colon cancer has been examined in a phase III randomised trial, the FoxTROT trial, and the final results of this large randomised trial are awaited (9).
Following any curative treatment of colon cancer, recurrence may develop locoregionally (including within the peritoneal cavity) or systemically. Considerable improvements have been made in the management and outcomes of patients with recurrent colorectal cancer in recent decades as a result of refined surgical techniques and improvements in radiation technology and chemotherapy regimens. This article summarises recent developments and future directions in the multimodal management of systemic and LR of colon cancer.
Locoregional recurrence
LR has been documented in 4–11.5% of patients after curative resection for colon cancer (10-12). LR develops from residual tumour cells not excised at the time of surgery, either at the colonic margin, in the draining lymphatic vessels or nodes, or due to direct extension or spillage into the peritoneal cavity. Factors that have been associated with an increased risk of developing LR in patients with colon cancer are more advanced disease stage or tumour grade, lymphovascular invasion, involved resection margins, left sided tumours and where the tumour causes obstruction, perforation, invasion of adjacent structures (11-13). Adjuvant chemotherapy has been associated with lower rates of LR in some patients with colon cancer (e.g. stage II or stage III tumors in one study), but this has not been a consistent finding (11,12).
LR has previously been categorised as peri-anastomotic, mesenteric/paracolic (local lymph node disease), retroperitoneal (loco-regional lymph node disease) and peritoneal (12,14,15). Approximately 20% of patients have more than one site of recurrence (14). Contemporary data indicates that the peritoneum is the most common site of LR (46%), which is considered separately due to the unique and evolving management options available to this group of patients (see below).
Detection and assessment
LR after curative resection is often more difficult to diagnose than might be expected. Like rectal cancer, recurrent colonic tumours may not have an intra-luminal component and therefore may not present with features of blood loss or obstruction. More often, symptoms are vague if present at all and may include lethargy and fatigue, abdominal pain and weight loss. Serum carcinoembryonic antigen is elevated in approximately 50% of patients with LR (14). Surgeons and oncologists must maintain a low threshold to further investigate patients with imaging, particularly within the first three years of surgery when the risk of LR is highest (12). Cross sectional imaging with a CT of the chest, abdomen and pelvis is generally the initial investigation of choice in patients with suspected LR, which may manifest as an intra-abdominal peri-anastomotic mass, retroperitoneal lymphadenopathy or with peritoneal metastases, characterized by omental nodularity, scalloping of the liver and ascites. 18F-fluorodeoxyglucose positron emission tomography (PET) combined with CT is now established as a standard part of assessment of all patients with recurrent colorectal cancer, in particular those who may be candidates for a potentially curative multi-modal management approach (16-19). There is some data to suggest PET-CT can identify small metastases not detected with CT alone (20).
All patients with LR of colon cancer should undergo colonoscopy as those with anastomotic recurrences may have an intra-luminal tumour component which is amenable to biopsy. Colonoscopy also excludes synchronous lesions and is therefore important even in patients with extra-luminal recurrence. Where there is a suspicion of mesenteric nodal or retroperitoneal recurrence on imaging and the lesions are FDG-avid, these factors are virtually diagnostic and, particularly in the setting of a raised CEA, does not require percutaneous biopsy (21). Diagnostic laparoscopy is the best investigation to confirm suspected peritoneal recurrence as it allows the surgeon to assess both the extent of peritoneal disease and obtain tissue for histopathology. For these reasons at our unit we have a low threshold to perform a laparoscopy in patients with any suspicion of peritoneal recurrence.
Management
Unlike locally recurrent rectal cancer, in which there has been increasing interest in the last decade and considerable progress has been made with multi-modal management strategies, there is relatively limited data to guide management of LR of colon cancer (Table 1). At the time of LR diagnosis, approximately 40% of patients will also have distant metastatic disease, while 60% of patients have isolated disease (12). The situation becomes incurable in patients with LR in the presence of unresectable metastatic disease, and a palliative approach is adopted, as discussed in the previous sections.
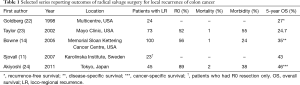
Full table
Patients with isolated peri-anastomotic or limited nodal recurrence in the mesentery or retroperitoneum may be potentially curative by radical resection in addition to adjuvant chemotherapy (14,23,24). For right sided recurrent tumours we position patients in the supine position, while for left sided and pelvic tumours patients are positioned in a modified Lloyd-Davies position to allow access to the pelvis. In general, patients with recurrent tumours require an open approach and, after a midline laparotomy is performed, a meticulous adhesiolysis is performed with care taken to avoid inadvertent enterotomies. The aim of salvage surgery for LR of colon cancer is segmental colectomy including re-excision of the anastomosis and residual mesentery with clear resection margins. Where there is local infiltration of the abdominal wall or adjacent organs, then radical en bloc resection is necessary in order to achieve a R0 resection. For tumours of the splenic flexure, this may involve partial gastrectomy, splenectomy or distal pancreatectomy. For recurrent right sided colon tumours, an en bloc pancreaticoduodenectomy may be necessary where there is tumour infiltration of the duodenum or head of the pancreas. In a recent report by Akiyoshi and colleagues including 45 patients who underwent salvage surgery for locally recurrent colon cancer, four patients (9%) required en bloc pancreaticoduodenectomy and two (4%) required pelvic exenteration (24). These more radical operations generally require input from hepatobiliary or oesophagogastric specialty colleagues for assistance with the resection and reconstruction, and a vascular surgeon may be required for tumours involving major retroperitoneal vessels. These more complex procedures be associated with increased morbidity and requires discussion during the informed consent process.
Table 1 summarizes selected reports including the perioperative and survival outcomes of patients undergoing radical surgery for LR of colon cancer. A large study by Bowne and colleagues at the Memorial Sloan Kettering Cancer Centre included 100 patients and reported an R0 resection rate of 56% which translated to a 35% disease-specific survival and was achieved with acceptable morbidity (24%) and mortality (1%) (14). Morbidity following salvage surgery may be substantial, and has been reported in 24–55% of patients and is likely higher when multivisceral resection are required.
Prevention strategies
Complete mesocolic excision (CME) with central vein ligation (CVL) for resection of right sided colon cancer is equivalent to the total mesorectal excision (TME) technique for rectal cancer. TME is based on dissection in the embryological and avascular mesorectal plane which avoids disruption of the draining lymphatics channels and allows complete resection of the peri-rectal (mesorectal) lymph node package, and has led to dramatic improvements in oncological outcomes for patients with rectal cancer. CME with CVL has more recently been proposed as a standardised technique for resection of colon cancer (25). Ligation of the inferior mesenteric artery at its origin from the aorta is considered a standard approach to resection of left sided colon cancer. For a right hemicolectomy, CME involves dissecting between the right mesocolon and the parietal peritoneum of the posterior abdominal wall, in order to access the ileocolic vessels are their origin. The mesocolon and vessels are divided along the superior mesenteric vein (SMV), in order to achieve complete resection of the lymphatic channels and lymph nodes anterior and lateral to the SMV (a D3 lymphadenectomy).
Although early data suggested that CME was associated with a lower rate of loco-regional recurrence when compared to conventional colectomy (25), it has not been universally taken up due to a lack of randomised data, and concerns about the technical difficulty and safety of this approach when performed laparoscopically or robotically. More recent data from a pooled analysis of conventional colectomy vs. CME demonstrated that CME improved disease-specific survival and was associated with lower rates of local recurrence (26) and furthermore laparoscopic CME has been shown to have equivalent oncological outcomes to open surgery without similar morbidity (27). On the basis of this more recent data, the authors would suggest that CME with CVL is an effective strategy to reduce the risk of LR during colectomy for colon cancer and can be feasibly performed laparoscopically.
Peritoneal recurrence
The risk of metachronous peritoneal metastasis (or peritoneal recurrence, PR) after curative resection of colorectal cancer has been reported as 3.4–6% in unselected cohorts (28-30) (Figure 1). There are, however, major difficulties in establishing the true incidence of PR relating to the poor sensitivity of contemporary imaging modalities for detecting peritoneal disease, limitations of administrative datasets and biased historical data (31). These issues make it difficult to define risk factors for PR, however there is evidence to suggest that limited synchronous peritoneal or ovarian metastasis which were resected at the index operation, perforated tumours, mucinous subtype and T4 tumours convey an increased risk of recurrent PM (28,29,32-34). Synchronous PM, synchronous ovarian metastasis and perforated tumours confer the greatest risk of developing PM after curative resection with reports in the literature as high as 27–71% (32).
Management
Traditionally PM carried a poor prognosis. Older systemic chemotherapy regimens based on 5-fluorouracil have been associated with a median survival of 5–7 months (35-37). This has improved with newer agents such as oxaliplatin, leucovorin, irinotecan and bevacizumab but treatment with adjuvant chemotherapy alone remains palliative treatment because of the limited penetration of peritoneal surfaces by systemic chemotherapy agents.
The application of cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC), initially developed in the 1990s, to colorectal PM has transformed the management and prognosis for this group of patients who now may be offered a chance of long-term survival. CRS is a radical procedure that aims to clear the peritoneal cavity of all peritoneal disease.
CRS usually comprise of stripping of the parietal peritoneum and omentectomy but may also extend to include visceral resections (splenectomy, partial gastrectomy, small bowel resection and colectomy) depending on the extent and location of the PM (38). In female patients, the ovaries are commonly affected by peritoneal disease and will manifest as Krukenberg tumours. These tumours do not tend to respond well to chemotherapy and can cause marked symptoms (Figure 2). Bilateral salpingo-oophorectomy is usually needed even if they appear macroscopically normal because of risk of microscopic involvement which is not as yet clinically apparent. Young women who have not completed child-bearing need to be appropriately counselled about this. Following CRS, a chemotherapy solution (generally high dose mitomycin or oxaliplatin diluted in 2–3 liters of crystalloid) is heated to 40–42 degree Celsius and circulated through the peritoneal cavity using a closed perfusion circuit driven by a rollator pump. Most patients will also receive concurrent systemic chemotherapy intra-operatively. This treatment strategy, in combination with adjuvant systemic chemotherapy, had resulted in reported median overall survival of 22–47 months and a 5-year survival of 26–58% in selected patients (39).
The peritoneal carcinomatosis index (PCI, range 0–39) is a staging system that defines the volume of peritoneal disease based on the size of peritoneal metastasis lesions within 13 defined areas in the peritoneal cavity. The Completeness of Cytoreduction (CC) Score defines the extent of disease remaining after CRS & HIPEC where CC-0 implies that there is no remaining visible disease. The completeness of CRS is well established as a prognostic indicator (40,41) and the ability of the surgeon to completely clear the peritoneal cavity is strongly influenced on the PCI score (i.e., the extent of PM within the peritoneal cavity). In addition to difficulty with complete cytoreduction with higher PCI scores, the survival benefit when the PCI is greater than 15 is not well established. Both of these factors have led to an increased interest in early detection or prevention of PR.
Early detection
Low volume colorectal PM is difficult to detect on routine imaging during follow up after colorectal cancer resection. Consequently, patients are often diagnosed late when the disease is visible on imaging or when symptoms develop. This necessarily means that the disease is more advanced with higher volume PM (i.e., higher PCI). It is also established that CT and PET imaging tend to understage the extent of disease and when patients are identified later, the burden of disease is often too advanced (PCI of >15) to offer any survival advantage with CRS & HIPEC, leaving patients with palliative systemic chemotherapy as the only treatment option.
The difficulties with early detection of disease and a lack of curative intent treatment options when disease is clinically evident have prompted the development of novel approaches which attempt to either prevent or identify PR at a subclinical stage in patients who are at high risk of developing PM. In a landmark study by Elias et al., 41 asymptomatic patients at high risk of colorectal PM (perforated tumours, resected low PM at time of primary colorectal cancer surgery and resected ovarian metastases) were enrolled prospectively to undergo a relook laparotomy at 12 months (42). The study found PM in 55% of their patients all of whom subsequently underwent CRS and HIPEC. The 2- and 5-year overall survivals of these patients were found to be 81% vs. 65% and 55% vs. 13% in the CRS + HIPEC group vs. the standard group respectively. Unfortunately the ProphyloCHIP trial, which randomised 150 patients at high risk of PR to standard surveillance vs. routine exploratory laparotomy, showed that second-look laparotomy was not associated with improved disease-free survival or PR (43) and therefore this approach has not been adopted outside of the trial setting.
Prophylactic HIPEC, where patients at high risk of PR are given HIPEC at the time of primary cancer surgery and in the absence of macroscopic PM, has been advocated by some authors as a potential way to reduce rates of PR. Similar to second-look surgery, there were encouraging early data to support the use of prophylactic HIPEC to reduce rates of PR. Randomised data from the COLOPEC trial became available in 2019, which was conducted across nine Dutch centres and randomised 204 patients with T4 or perforated tumours to standard resection and standard adjuvant systemic chemotherapy with or without HIPEC at the time of surgery (44). The results of this trial showed there was no difference in peritoneal-free survival at 18-month between the groups (80.9% for the experimental group vs. 76.2% for the control group, P=0.28). Therefore, like second-look surgery, this approach has not been broadly adopted. There are several ongoing trials addressing the issue of early detection and prevention of PR (45,46).
Liver metastases
Liver metastases can occur in up to 50% of patients following curative colon cancer treatment and is the most common site of recurrent disease. Traditionally, the approach to hepatic metastases was one of palliative chemotherapy as metastases were thought to be uniformly fatal. In the 1990s, Scheele et al. demonstrated favourable survival with liver resection (47) and in the two decades since, not only liver resection has become widely accepted, the boundaries of what constitutes resectable liver metastases has also changed dramatically. Initially surgeons adhered to anatomically defined criteria (number of metastases, size of metastases, uni vs. bilobar disease) and the need for a 1cm resection margin, but in contemporary practice resectability is defined by the ability to excise all disease with a clear margin and an adequate remnant liver (48). Liver resection has been shown to improve 5 year overall survival from <5% to up to 60% in contemporary series (49-51) (Table 2).

Full table
Resectability criteria
In contemporary practice, resectability of colorectal liver metastases is evaluated in a multi-disciplinary setting and according to three domains (medical, oncological and technical resectability) in order to optimise patient selection and ensure safe and effective liver resections (48). Medical resectability includes evaluation of chronic comorbidities and the degree of underlying hepatic impairment. Oncological resectability includes consideration of the primary tumour characteristics including its molecular profile, resection margins and nodal status, the number and size of hepatic lesions, the presence of extrahepatic disease, as well as the length of disease-free interval in patients.
Technical resectability
Technical resectability is defined by complete oncological excision of all metastases (i.e., R0 resection) with an adequate functional liver remnant. Generally, this requires preservation of two or more liver segments with intact vascular inflow, outflow, and biliary drainage. An assessment of resectability depends on both hepatic imaging to estimate the volume of the future liver remnant as well as the variety of surgical techniques that can be utilised, which is beyond the scope of this article.
It is clear that a negative surgical margin is an important factor in long-term survival for patients undergoing hepatectomy. Scheele et al. demonstrated that patients with a microscopically or macroscopically involved margin resection had worse survival compared to those who had a clear resection margin (median survival of 14 vs. 40 months) (47). However, what constitutes a clear margin is more controversial. Older studies have reported worse survival with margins <1 cm (57,58), but more recent studies have refuted this (51,59). A recent meta-analysis included 11,147 hepatic resections in 34 studies found that resection margin >1 cm was associated with improved 3-, 5- and 10-year overall and disease-free survival (60). On the basis of this data, most units would currently consider a clear margin >1 cm as the ultimate goal of resection, however a narrower margin is not in itself a contraindication for surgery (61).
Future liver remnant
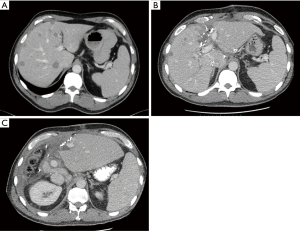
With improved imaging, chemotherapeutic agents including the introduction of biological therapy and improved operative techniques, criteria for resectability is now extended such that the focus is hepatic insufficiency. In view of this, and in order to increase the pool of patients eligible for surgery, there has been considerable interest in increasing the future liver remnant (FLR). In general, a 20% or greater FLR is adequate in patients with normal underlying liver parenchyma. A higher FLR however is needed in patients on chemotherapy because of concerns for steatohepatitis induced by chemotherapy (>30%) and even higher in patients with cirrhosis (>40%). If the FLR is anticipated to be inadequate, then techniques to induce hypertrophy of the future remnant include staged resections, the associating liver partition and portal vein ligation for staged hepatectomy procedure, and portal vein embolization (PVE). Preoperative PVE is the most common approach and involves occlusion of portal perfusion to the part of the liver planned for resection. This is performed several weeks prior to surgery, which induces hypertrophy of the expected FLR, and may make more extensive hepatectomies feasible, particularly for right sided resections (62). This may also be combined with other techniques to result in more rapid liver hypertrophy. One recent technique that is novel and promising is ALPPS (Associated Liver Partitioning and Portal Vein ligation for staged hepatectomy) (Figure 3).
Parenchymal-sparing vs. anatomical resection techniques
Anatomical (i.e., segmental) resections have traditionally been preferred for colorectal liver metastases, however there is increasing use of non-anatomical (or parenchymal-sparing) resections. A recent meta-analysis demonstrated that parenchymal-sparing techniques do not compromise oncological or survival outcomes when compared to anatomical resections, and are not associated with higher rates of postoperative complications, length of stay or intraoperative blood loss (63). Importantly, parenchymal-sparing techniques preserves liver function, an important consideration in a group of patient who have generally had previous systemic chemotherapy, and may increase the feasibility of re-resection in the case of hepatic re-recurrence.
Imaging
The aims of imaging during the assessment of hepatic metastases are to define the number and anatomical location of lesions, proximity to major vascular and biliary structures, and to identify extra-hepatic disease. High resolution CT with contrast enhancement is a readily available and low-cost modality with reported sensitivity and specificity rates of between 70–95% and 96%, respectively, for the detection of hepatic metastases. CT is, however, limited in its ability to identify lesions smaller than 1cm as well as extrahepatic disease. For these reasons, MRI with hepatospecific contrast enhancement (e.g., gadoxetate disodium, tradename PrimovistTM) is now established as the imaging of choice at specialist units for the evaluation of patients for potentially curative liver resection. MRI has demonstrated comparatively better detection rates for sub-centremetre lesions, in particular in patients with hepatic steatosis (which is common following systemic chemotherapy) and higher soft tissue definition may allow better assessment of infiltration of major hepatic vascular and biliary structures (64,65). The role of PET as part of the evaluation of patients with colorectal liver metastases is unclear. While some data has demonstrated a reduction in futile laparotomies by detection of occult extra-hepatic metastases on preoperative PET (66), results from other studies have been conflicting (67). Importantly, FDG-avidity of hepatic lesions is difficult to interpret in the setting of recent systemic chemotherapy, which may cause lesions to become metabolically “switch off” for several weeks after chemotherapy, with reported false negative rates for detection of hepatic metastases as high as 87% (68).
Neoadjuvant chemotherapy
In patients with resectable liver disease, the role of pre-operative chemotherapy is unclear. While it makes biological sense for patients to receive chemotherapy for management of micrometastatic disease, chemotherapeutic agents, particularly modern chemotherapy agents can lead to liver injury, increasing peri-operative morbidity and mortality and data examining these considerations is conflicting. In a randomised trial comparing peri-operative FOLFOX plus liver resection surgery versus liver resection alone did not demonstrate an overall survival benefit although there was an improvement in progression free survival rates at 3 years (69,70). Another potential role of chemotherapy is in some patients with initially unresectable or borderline resectable metastases, where a period of systemic chemotherapy may ‘convert’ them to resectable disease or act as a measure of tumour biology before hepatectomy is considered.
There is ongoing debate surrounding the use of systemic chemotherapy in patients with potentially resectable hepatic metastases and how it might be best integrated with surgery. At our institution patients who are medically well with low volume, potentially resectable disease generally undergoing upfront surgery followed by adjuvant chemotherapy. Those with high volume, borderline resectable disease or who are poor candidates for surgery from a medical fitness point of view generally undergo an initial period of systemic chemotherapy, after which they are reassessed as to whether potentially curative resection is possible.
Incurable liver metastases
The majority of patients with metachronous hepatic metastases from colorectal cancer, even in the absence of extra-hepatic recurrence, do not have potentially curative disease due to tumour location or due to poor hepatic reserve. In this group of patients, modern chemotherapy regimens that include oxaliplatin or irinotecan have dramatically improved survival such that median survivals of 30 months have been reported (71). In addition to systemic therapy, for patients whose disease progress while on systemic therapy, a number of local and regional approaches have been developed, including radiofrequency and microwave ablation, hepatic intra-arterial, and various radiotherapy, however data supporting a survival advantage for most of these therapies is limited.
Pulmonary metastases
Pulmonary metastases are the second most common site for systemic recurrence after the liver. These metastases may also be amendable to resection. However, as only 10% who develop pulmonary metastases will have the disease confined to the lung, few patients will be suitable for potentially curative surgery. Studies reporting pulmonary metastasectomy outcomes therefore reflect a highly select group of patients with favourable disease (72). In these studies, pooled 5-year overall survivals of up to 40% have been reported, which would certainly seem to favour pulmonary metastasectomy (73). However, it is also important to understand that lung metastases are generally asymptomatic and an uncommon cause of death in patients with metastatic colorectal cancer. The true value of pulmonary metasectomy is therefore unclear and it is on this premise that the PULMICC (Pulmonary Metasectomy in Colorectal Cancer) trial was designed (74). The trial is a 2-armed multicentre randomised trial which compared pulmonary metastasectomy with continued observation. After prolonged and slow accrual from 2010 to 2016, the trial was terminated early in 2016 after enrolling 65 patients (sample size of 300 at trial inception). The 4-year overall survival were 40% (95% CI: 26–63%) for the control group versus 43% (95% CI: 27–66%) for patients assigned to metastasectomy. At 5 years, the estimated survival was 29% (95% CI: 16–52%) and 38% (95% CI: 23–62%) for control and metastasectomy groups respectively. Although this trial was not able to inform the survival benefit of metastasectomy, what it did confirm is that patients with lung metastases can have good survival outcomes without surgery, which contrasts with the widely held assumption that patients with lung metastases have a 5-year overall survival of <5% (74).
In patients with unresectable lung metastases, ablative techniques are commonly offered as an alternative to lung resection. The assumption associated with ablative techniques is that it may offer the same survival benefit but with less surgical morbidity because of the minimally invasive nature of the technique. A number of ablative techniques are available including radiofrequency ablation, image guided thermal ablation, microwave ablation and stereotactic ablative radiotherapy amongst others (75). The common principle of ablative techniques is to apply an energy source to the metastasis such that the cancer tissue is destroyed (ablated) regardless of the energy source used. The delivery is aimed at the centre of the lesion which causes irreversible cellular damage and hence immediate tissue destruction (76). Surrounding this however, will be a zone of lethal exposure but the energy delivery is such that tissue death is imminent rather than being immediate. Beyond this, there is usually non-lethal cell injury, where inflammatory process usually results in increased blood flow and a rim of hyperaemia. A concern with ablative techniques, despite its minimally invasive nature is the lack of tissue for histology and hence the inability to confirm if the lesion has been completely ablated with a normal “margin”. At present, no ablative technique has been adequately assessed in a randomised trial to allow its effectiveness to be determined (75).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nan Zun Teo and James Chi-Yong Ngu) for the series “Current Strategies in Colon Cancer Management” published in Journal of Gastrointestinal Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (Available online: http://dx.doi.org/10.21037/jgo-2019-ccm-09). The series “Current Strategies in Colon Cancer Management” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1.0. Lyon, France: International Agency for Research on Cancer 2012. Available online: https://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOBOCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-2012
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683-91. [Crossref] [PubMed]
- Bowel cancer (Colorectal cancer) in Australia statistics: Cancer Australia. Available online: https://bowel-cancer.canceraustralia.gov.au/statistics
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Kalady MF, Sanchez JA, Manilich E, et al. Divergent oncogenic changes influence survival differences between colon and rectal adenocarcinomas. Dis Colon Rectum 2009;52:1039-45. [Crossref] [PubMed]
- Benson AB, Venook AP, Al-Hawary MM, et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw 2018;16:359-69. [Crossref] [PubMed]
- Sebastian NT, Tan Y, Miller ED, et al. Surgery With and Without Adjuvant Radiotherapy Is Associated With Similar Survival in T4 Colon Cancer. Colorectal Dis 2020. [Epub ahead of print].
- Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012;13:1152-60. [Crossref] [PubMed]
- Read TE, Mutch MG, Chang BW, et al. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg 2002;195:33-40. [Crossref] [PubMed]
- Sjövall A, Granath F, Cedermark B, et al. Loco-regional recurrence from colon cancer: a population-based study. Ann Surg Oncol 2007;14:432-40. [Crossref] [PubMed]
- Liska D, Stocchi L, Karagkounis G, et al. Incidence, Patterns, and Predictors of Locoregional Recurrence in Colon Cancer. Ann Surg Oncol 2017;24:1093-9. [Crossref] [PubMed]
- Elferink MA, Visser O, Wiggers T, et al. Prognostic factors for locoregional recurrences in colon cancer. Ann Surg Oncol 2012;19:2203-11. [Crossref] [PubMed]
- Bowne WB, Lee B, Wong WD, et al. Operative salvage for locoregional recurrent colon cancer after curative resection: an analysis of 100 cases. Dis Colon Rectum 2005;48:897-909. [Crossref] [PubMed]
- Landmann RG, Weiser MR. Surgical management of locally advanced and locally recurrent colon cancer. Clin Colon Rectal Surg 2005;18:182-9. [Crossref] [PubMed]
- Bird TG, Ngan SY, Chu J, et al. Outcomes and prognostic factors of multimodality treatment for locally recurrent rectal cancer with curative intent. Int J Colorectal Dis 2018;33:393-401. [Crossref] [PubMed]
- You YN, Skibber JM, Hu CY, et al. Impact of multimodal therapy in locally recurrent rectal cancer. Br J Surg 2016;103:753-62. [Crossref] [PubMed]
- Mirnezami AH, Sagar PM, Kavanagh D, et al. Clinical algorithms for the surgical management of locally recurrent rectal cancer. Dis Colon Rectum 2010;53:1248-57. [Crossref] [PubMed]
- Bhangu A, Ali SM, Cunningham D, et al. Comparison of long-term survival outcome of operative vs. nonoperative management of recurrent rectal cancer. Colorectal Dis 2013;15:156-63. [Crossref] [PubMed]
- Watson AJ, Lolohea S, Robertson GM, et al. The role of positron emission tomography in the management of recurrent colorectal cancer: a review. Dis Colon Rectum 2007;50:102-14. [Crossref] [PubMed]
- Dozois EJ, Colibaseanu DT. Colorectal Cancer: Management of Local recurrence. In: The ASCRS Textbook of Colon and Rectal Surgery. New York: Springer Publishing Company, 2016
- Goldberg RM, Fleming TR, Tangen CM, et al. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection. Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med 1998;129:27-35. [Crossref] [PubMed]
- Taylor WE, Donohue JH, Gunderson LL, et al. The Mayo Clinic experience with multimodality treatment of locally advanced or recurrent colon cancer. Ann Surg Oncol 2002;9:177-85. [Crossref] [PubMed]
- Akiyoshi T, Fujimoto Y, Konishi T, et al. Prognostic factors for survival after salvage surgery for locoregional recurrence of colon cancer. Am J Surg 2011;201:726-33. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis 2009;11:354-64; discussion 364-5. [Crossref] [PubMed]
- Alhassan N, Yang M, Wong-Chong N, et al. Comparison between conventional colectomy and complete mesocolic excision for colon cancer: a systematic review and pooled analysis. Surg Endosc 2019;33:8-18. [Crossref] [PubMed]
- Athanasiou CD, Markides GA, Kotb A, et al. Open compared with laparoscopic complete mesocolic excision with central lymphadenectomy for colon cancer: a systematic review and meta-analysis. Colorectal Dis 2016;18:O224-35. [Crossref] [PubMed]
- van Gestel YR, Thomassen I, Lemmens VE, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol 2014;40:963-9. [Crossref] [PubMed]
- Quere P, Facy O, Manfredi S, et al. Epidemiology, Management, and Survival of Peritoneal Carcinomatosis from Colorectal Cancer: A Population-Based Study. Dis Colon Rectum 2015;58:743-52. [Crossref] [PubMed]
- Nagata H, Ishihara S, Hata K, et al. Survival and Prognostic Factors for Metachronous Peritoneal Metastasis in Patients with Colon Cancer. Ann Surg Oncol 2017;24:1269-80. [Crossref] [PubMed]
- Jacobson R, Sherman SK, Dahdaleh F, et al. Peritoneal Metastases in Colorectal Cancer. Ann Surg Oncol 2018;25:2145-51. [Crossref] [PubMed]
- Honoré C, Goere D, Souadka A, et al. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol 2013;20:183-92. [Crossref] [PubMed]
- Hugen N, van de Velde CJ, de Wilt JH, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014;25:651-7. [Crossref] [PubMed]
- Segelman J, Akre O, Gustafsson UO, et al. External validation of models predicting the individual risk of metachronous peritoneal carcinomatosis from colon and rectal cancer. Colorectal Dis 2016;18:378-85. [Crossref] [PubMed]
- Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545-50. [Crossref] [PubMed]
- Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989;63:364-7. [Crossref] [PubMed]
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [Crossref] [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [Crossref] [PubMed]
- Hall B, Padussis J, Foster JM. Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy in the Management of Colorectal Peritoneal Metastasis. Surg Clin North Am 2017;97:671-82. [Crossref] [PubMed]
- Faron M, Macovei R, Goere D, et al. Linear Relationship of Peritoneal Cancer Index and Survival in Patients with Peritoneal Metastases from Colorectal Cancer. Ann Surg Oncol 2016;23:114-9. [Crossref] [PubMed]
- Elias D, Faron M, Iuga BS, et al. Prognostic similarities and differences in optimally resected liver metastases and peritoneal metastases from colorectal cancers. Ann Surg 2015;261:157-63. [Crossref] [PubMed]
- Elias D, Honore C, Dumont F, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg 2011;254:289-93. [Crossref] [PubMed]
- Goere D, Glehen O, Quenet F, et al. Results of a randomized phase 3 study evaluating the potential benefit of a second-look surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-NTC01226394). J Clin Oncol 2018;2018;36:abstr 3531.
- Klaver CEL, Wisselink DD, Punt CJA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol 2019;4:761-70. [Crossref] [PubMed]
- Arjona-Sánchez A, Barrios P, Boldo-Roda E, et al. HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer 2018;18:183. [Crossref] [PubMed]
- Proactive management of endoperitoneal spread in colonic cancer. PROMENADE trial (NCT 02974556). Available online: https://clinicaltrials.gov/ct2/show/NCT02974556
- Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995;19:59-71. [Crossref] [PubMed]
- Marcus RK Aloia TA. Defining Resectability of Colorectal Cancer Liver Metastases: Technical and Oncologic Perspectives. In: Correia MM, Choti MA, Rocha FG, et al. (editors). Colorectal Cancer Liver Metastases. Springer, 2020.
- Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35. [Crossref] [PubMed]
- Booth CM, Nanji S, Wei X, et al. Surgical resection and peri-operative chemotherapy for colorectal cancer liver metastases: A population-based study. Eur J Surg Oncol 2016;42:281-7. [Crossref] [PubMed]
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715-22, discussion 722-4. [Crossref] [PubMed]
- Wei AC, Greig PD, Grant D, et al. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 2006;13:668-76. [Crossref] [PubMed]
- Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer 2007;109:718-26. [Crossref] [PubMed]
- Robertson DJ, Stukel TA, Gottlieb DJ, et al. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer 2009;115:752-9. [Crossref] [PubMed]
- Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110-8. [Crossref] [PubMed]
- Zaydfudim VM, McMurray TL, Harrigan AM, et al. Improving treatment and survival: a population-based study of current outcomes after a hepatic resection in patients with metastatic colorectal cancer. HPB 2015;17:1019-24. [Crossref] [PubMed]
- Cady B, Jenkins RL, Steele GD, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg 1998;227:566-71. [Crossref] [PubMed]
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer 1996;77:1254-62. [Crossref] [PubMed]
- Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg 2007;246:295-300. [Crossref] [PubMed]
- Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, et al. Impact of Surgical Margin Width on Recurrence and Overall Survival Following R0 Hepatic Resection of Colorectal Metastases: A Systematic Review and Meta-analysis. Ann Surg 2018;267:1047-55. [Crossref] [PubMed]
- Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1261-8. [Crossref] [PubMed]
- Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386-94. [Crossref] [PubMed]
- Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, et al. Parenchymal-Sparing Versus Anatomic Liver Resection for Colorectal Liver Metastases: a Systematic Review. J Gastrointest Surg 2017;21:1076-85. [Crossref] [PubMed]
- Asato N, Tsurusaki M, Sofue K, et al. Comparison of gadoxetic acid-enhanced dynamic MR imaging and contrast-enhanced computed tomography for preoperative evaluation of colorectal liver metastases. Jpn J Radiol 2017;35:197-205. [Crossref] [PubMed]
- Scharitzer M, Ba-Ssalamah A, Ringl H, et al. Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur Radiol 2013;23:2187-96. [Crossref] [PubMed]
- Ruers TJ, Wiering B, van der Sijp JR, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with (18)F-FDG PET: a randomized study. J Nucl Med 2009;50:1036-41. [Crossref] [PubMed]
- Moulton CA, Gu CS, Law CH, et al. Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA 2014;311:1863-9. [Crossref] [PubMed]
- Glazer ES, Beaty K, Abdalla EK, et al. Effectiveness of positron emission tomography for predicting chemotherapy response in colorectal cancer liver metastases. Arch Surg 2010;145:340-5; discussion 345. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208-15. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Treasure T. Pulmonary Metastasectomy for Colorectal Cancer: Recent Reports Prompt a Review of the Available Evidence. Curr Colorectal Cancer Rep 2014;10:296-302. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Treasure T, Farewell V, Macbeth F, et al. Pulmonary Metastasectomy versus Continued Active Monitoring in Colorectal Cancer (PulMiCC): a multicentre randomised clinical trial. Trials 2019;20:718. [Crossref] [PubMed]
- Treasure T. Surgery and ablative techniques for lung metastases in the Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) trial: is there equivalence? J Thorac Dis 2016;8:S649-51. [Crossref] [PubMed]
- Diederich CJ. Thermal ablation and high-temperature thermal therapy: overview of technology and clinical implementation. Int J Hyperthermia 2005;21:745-53. [Crossref] [PubMed]