A phase II study of biweekly pralatrexate and docetaxel in patients with advanced esophageal and gastroesophageal carcinoma that have failed first-line platinum-based therapy
Introduction
Esophageal (E) and gastroesophageal (GE) cancers are among the most common neoplasms, with an estimated 40,390 new cases and 26,550 deaths expected in the United States in 2014 (1). Treatment options for patients with relapsed or refractory disease remain limited, and despite improvements in palliative chemotherapy and biologic agents, partial response (PR) rates remain between 25% and 50%, with overall survival (OS) measured in months (2-4).
Methotrexate in combination with 5-fluoruracil and doxorubicin (FAMTX) was considered a standard therapy for metastatic E and GE cancers before being supplanted by platinum-based regimens (5). Pralatrexate is a 10-deaza analog of methotrexate, but with improved cellular uptake and retention (6).
Docetaxel has proven efficacy in gastric, E, and GE tumors (5). A recent phase III randomized trial (COUGAR-02) of patients with refractory GE cancers reported an improvement in outcome with docetaxel vs. active symptom control alone (7). Docetaxel improved median OS to 5.2 months (95% CI, 4.1-5.9), as compared to 3.6 months (95% CI, 3.3-4.4) in the active symptom control arm. Overall response rate (ORR) was 7% and 43% patients had progressive disease (PD) in the docetaxel arm. Previously, a phase III randomized study of patients with untreated, advanced gastric or GE adenocarcinoma received either docetaxel, cisplatin and fluorouracil (DCF) or docetaxel and cisplatin (DC) every 3 weeks (8). The ORR was 43% for DCF and 26% for DC.
Regimens of pralatrexate combined with either paclitaxel or docetaxel have been studied in non-small cell lung cancer and demonstrated promising activity (9). Given all of the above, we hypothesized that pralatrexate and docetaxel would be efficacious for the treatment of refractory E and GE cancers. We conducted a phase II study of biweekly pralatrexate and docetaxel in patients with advanced E or GE carcinoma whom have failed prior platinum-based therapies.
Methods
Eligible patients were required to have unresectable or metastatic carcinoma of the E or GE junction. Other key inclusion criteria included established histologically-confirmed squamous cell carcinoma or adenocarcinoma, prior platinum-based therapy, measurable disease per Response Evaluation Criteria in Solid Tumors v1.1 (RECIST), life expectancy of at least 12 weeks, normal end organ function, and Eastern Cooperative Oncology Group (ECOG) performance scores of 0-1 (10). Patients were excluded if they had undergone more than three prior chemotherapy regimens, had received therapy within 4 weeks prior to enrollment, or had evidence of pleural effusion or ascites.
Study design
This was a phase II single-arm, open label trial performed at The Ohio State University James Cancer Center. The institutional review board approved the study, and the registered trial number is NCT01129206. This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by Allos Therapeutics Inc./Spectrum Pharmaceuticals. Informed consent was obtained from each subject, and all aspects of the study met the standards of the Declaration of Helsinki.
Treatment plan
Pralatrexate and docetaxel were administered on day 1 of a 14-day cycle. Pralatrexate 120 mg/m2 was administered by intravenous push, followed by docetaxel 35 mg/m2 via one-hour intravenous infusion. To prevent docetaxel hypersensitivity reactions, patients received dexamethasone 8 mg twice daily by mouth 3 days prior to day 1. Anti-emetics were administered per institution standard.
Assessment of response and toxicity
Radiological assessment of tumor response was performed by computed tomography (CT) and positron emission tomography (PET) every four cycles of therapy and responses were measured according to the RECIST and PERCIST criteria. Toxicities were defined by the NCI-CTCAE, version 3.0 (11).
Statistical methods
The primary end-point was the overall response rate [PR + complete response (CR)] following the initiation of pralatrexate plus docetaxel. We used a Fleming single-stage phase II trial design with a total of 32 evaluable patients with an alpha of 0.1 and a power of 0.9. Based on historic data, we considered the treatment to be ineffective if the ORR was less than 5% (3 or fewer responses were seen in a total of 32 patients) (p0). We assumed that the regimen was worthy of further study if the true ORR was 20% or greater (p1). The secondary end-points were PFS and OS. PFS was calculated from the date of start of therapy to disease progression or death, whichever occurred first. OS was determined from the date of start of therapy to death from any cause. Survival curves were estimated using the method of Kaplan-Meier.
Results
Patient characteristics
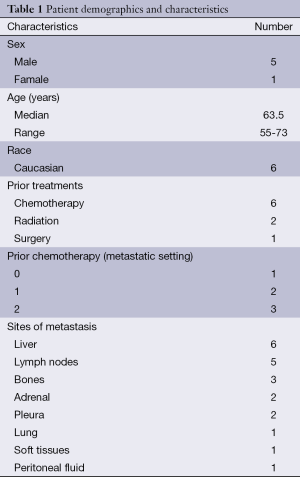
Six patients were accrued out of the 32 planned due to loss of grant funding. Five males and one female were enrolled. All tumors were adenocarcinoma. One patient had no prior chemotherapy in the metastatic setting, two patients had one prior therapy, and three patients had two prior lines of therapy (Table 1).
Full table
Treatment toxicity
The most common drug toxicities were lymphopenia (6 patients), anemia (2 patients), leukopenia (2 patients), and mucositis (2 patients). Grade 3 side effects were lymphopenia (4 patients), anemia (2 patients), mucositis (1 patient), and fatigue (1 patient). Two patients required dose reductions secondary to fatigue and mucositis (both grade 3). There were no grade 4 toxicities.
Treatment efficacy
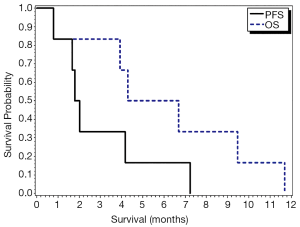
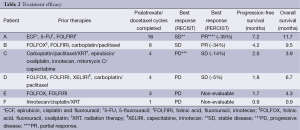
A mean of four cycles was administered (range 1-16). Two patients had stable disease (SD) and four patients had PD per RECIST criteria, yielding an ORR of 0%. When applying PERCIST criteria in four evaluable patients, two had a PR (SD per RECIST criteria) and two had SD (PD per RECIST criteria). The patients who were identified to have a PR had prolonged OS of 9.5 and 11.7 months. Median progression-free survival (PFS) was 1.9 months (95% CI, 0.8-7.2) and median OS was 5.5 months (95% CI, 0.8-11.7) (Table 2) (Figure 1).
Full table
Discussion
There remain very few standard options for E and GE cancers in patients who have failed first-line platinum-based therapy. Systemic chemotherapy options largely depend on the patient’s performance status, as to whether they are able to tolerate combination vs. single agent therapy. Relatively active cytotoxic agents include taxanes such as docetaxel or paclitaxel, irinotecan or 5-fluorouracil (5-FU). Recently, the results of the phase III COUGAR-02 trial suggested that patients with refractory GE adenocarcinoma receiving docetaxel vs active symptom control had an OS benefit of 1.6 months. Additional options have been made available to patients with metastatic GE or gastric cancer with the recent FDA approval of ramucirumab, a monoclonal antibody targeting vascular endothelial growth factor-receptor 2, either as a single-agent in the palliative setting or in combination with paclitaxel. In our study, and given the preliminary activity of pralatrexate and docetaxel in non-small cell lung cancers, and single agent docetaxel activity in E cancer, we set out to determine if the combination against E and GE cancer would be effective as well.
The phase II study of pralatrexate and paclitaxel or docetaxel in patients wth advanced solid tumors reported the maximum tolerated dose to be pralatrexate 120 mg/m2 every 2 weeks and docetaxel 35 mg/m2 every 2 weeks (12). Supplementation with Vitamin B12 and folate preceding the start of the combination chemotherapy allowed for higher doses of pralatrexate to be delivered safely and decreased the risk of stomatitis and neutropenia. We implemented vitamin supplementation in this trial, but we did see 2 of the 6 patients require dose-reductions due to grade 3 stomatitis and fatigue.
Our study showed that docetaxel and pralatraxate was a tolerable regimen, but in the six patients accrued, the combination therapy did show evidence of meaningful activity compared to historical control (7). It is certainly possible that the premature halt of the study may have affected this conclusion. However, a goal of 20% or more was unlikely to be reached in the absence of any responder in the first six patients, with both OS and PFS for the combination in line of what you would expect with single agent docetaxel. The survival rates in our study were comparable to patients with E cancers undergoing second-line therapies as previously reported with of PFS and OS rates ranging from 1.4-6.2 and 4.0-11.4 months, respectively (13).
Interestingly, our study, suggests that PERCIST response in combination with RECIST may better assess clinical disease response as compared to RECIST response alone, in refractory settings where a RECIST response may not necessarily be reflective of an improved outcome (14).
Our study was unfortunately halted prematurely. However, our limited analysis does not support the combination of pralatrexate and docetaxel in advanced E and GE cancers. It is uncertain if a larger number of subjects may prove to show improved outcome. We will await the results of a phase II study of pralatrexate in combination with oxaliplatin in patients with advanced esophagogastric cancer (NCT01178944) to determine any further developemnt of this agent in GE cancers.
Acknowledgements
Funding: This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by Allos Therapeutics Inc./Spectrum Pharmaceuticals. Christina Wu is funded on K12 Faculty Training Grant (CA133250).
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997;15:261-7. [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [PubMed]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. [PubMed]
- Wils JA, Klein HO, Wagener DJ, et al. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin--a step ahead in the treatment of advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol 1991;9:827-31. [PubMed]
- Wang ES, O'Connor O, She Y, et al. Activity of a novel anti-folate (PDX, 10-propargyl 10-deazaaminopterin) against human lymphoma is superior to methotrexate and correlates with tumor RFC-1 gene expression. Leuk Lymphoma 2003;44:1027-35. [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [PubMed]
- Ajani JA, Fodor MB, Tjulandin SA, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol 2005;23:5660-7. [PubMed]
- Azzoli CG, Krug L, Miller V, et al. Phase I study of the antifolate pralatrexate given with vitamin B12 and folic acid supplementation in patients (pts) with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2007;25:abstr 13006.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Available online: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed July 31, 2014.
- Azzoli CG, Krug LM, Gomez J, et al. A phase 1 study of pralatrexate in combination with paclitaxel or docetaxel in patients with advanced solid tumors. Clin Cancer Res 2007;13:2692-8. [PubMed]
- Thallinger CM, Raderer M, Hejna M. Esophageal cancer: a critical evaluation of systemic second-line therapy. J Clin Oncol 2011;29:4709-14. [PubMed]
- Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122S-50S. [PubMed]