Surgical management of hepatocellular carcinoma after Fontan procedure
The Fontan operation is used to separate the systemic and pulmonary circulations in patients born with single ventricle physiology. Systemic venous return is directed to the lungs via passive circulation without the aid of a sub-pulmonic ventricle. While the Fontan operation addresses the critical problems of cyanosis and volume overload in these patients, it necessitates chronic elevation in central venous pressure (CVP) to drive pulmonary circulation. Additionally, patients with Fontan circulation have a low hepatic perfusion pressure, chronic hypoxemia, and decreased cardiac output. These pathophysiological changes produce liver hypoperfusion (1), especially in response to metabolic demands, thus subjecting the liver to repeated rounds of injury and inflammation, leading to fibrosis and ultimately cirrhosis (1).
Due to significant advances in pediatric cardiology, survival of patients with single ventricle physiology has improved dramatically, with one study reporting greater than 80% of these patients to be alive without cardiac transplantation at 20 years (2). As these patients live to become adults, long-term sequelae of their chronic hemodynamic alterations are posing new challenges including complications of cirrhosis such as hepatocellular carcinoma (HCC) (3). The management of HCC in patients with post-Fontan physiology is not well described (4-6); to our knowledge this report is the first description of successful HCC resection of HCC in a patient with this cardiovascular anatomy.
Case presentation
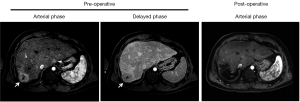
A 32-year-old male with history of double inlet left ventricle had undergone a right atrial to pulmonary artery Fontan operation at age 9 years, tricuspid valve patch at age 14 years, and catheter-directed ablation for reentrant tachycardia at age 18 years. On routine follow-up, he had an incidental finding of a liver mass on cardiac MRI. Subsequent multi-phase liver MRI showed a partially exophytic 4 cm liver mass arising from segment 7, suggestive of HCC (LiRads 5B) and a 1 cm hypervascular mass without washout in segment 1 (LiRads 3) (Figure 1). The liver parenchyma had a coarsened appearance consistent with cirrhosis, but there was no radiographic evidence of portal hypertension. Following reversal of his warfarin therapy, an ultrasound-guided needle biopsy of the segment 7 lesion demonstrated HCC of the fibrolamellar variant. No extra-hepatic disease was found on staging studies.
The patient had no other significant medical problems and had no other risk factors for cirrhosis; his liver function was well preserved with a total bilirubin of 1.1 milligrams/deciliter (dL), albumin of 4.8 grams/dL, aspartate aminotransferase of 23 international units/liter (IU/L), alanine aminotransferase of 21 IU/L, alkaline phosphatase of 72 IU/L, international normalized ratio of 2.2 (on warfarin for atrial arrhythmia). Other lab findings included an alpha-fetoprotein of 13 micrograms/liter (normal less than 40), platelet count of 225,000/microliter, and creatinine of 0.91 milligram/dL. An echocardiogram demonstrated normal ventricular function and no obstruction in the Fontan circuit; cardiopulmonary exercise testing demonstrated a V02 of 65% predicted. Patient’s arterial oxygen saturation on room air at rest was 96%. Cardiac catheterization was performed, revealing a CVP of 12-13 mmHg and a hepatic wedge pressure 15 mmHg, representing a normal portal-systemic gradient.
After an extensive multidisciplinary discussion, resection of his HCC was planned, and warfarin was stopped 5 days before the procedure with enoxaparin bridging. Prior to induction, a T6-7 epidural catheter and an arterial line were placed. He tolerated induction without significant hemodynamic fluctuations. A central venous catheter was inserted, and the patient underwent normo-volemic hemodilution to preserve 900 milliliters of autologous blood. Rather than ‘low-CVP anesthesia’ to minimize blood loss, we aimed to maintain right-sided filling pressures between 10-15 mmHg, based on pre-operative evidence that these pressures optimize his cardiac filling and output.
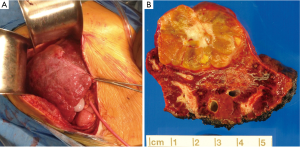
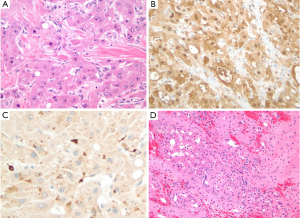
A right subcostal incision was performed, revealing a firm nodular liver consistent with cardiac cirrhosis (Figure 2). There was no evidence of extra-hepatic disease on abdominal exploration. We evaluated the patient’s tolerance of inflow occlusion with the Pringle maneuver, which led to a 1-2 mmHg transient drop in CVP without changing his systemic blood pressure. Intraoperative ultrasound confirmed a large lesion in segment 7 in the proximity of the right hepatic vein and a 1cm lesion in segment 1. Five minutes of Pringle occlusion was performed prior to parenchymal dissection per our standard ischemic pre-conditioning protocol. The right hepatic vein was isolated and the effect of its occlusion was tested with no hemodynamic consequence. A partial right hepatectomy was performed (segments 6 and 7), with parenchymal transection using a combination of an ultrasonic energy device and hydro-jet dissection. The segment 1 lesion was removed via a separate wedge resection. Estimated blood loss was 1.5 L, which was replaced with 1 unit of packed red blood cells in addition to the patient’s autologous blood, and he remained hemodynamically stable with adequate urine output throughout the procedure. Pathologic evaluation of the segment 7 lesions revealed gross margins of ~2 cm and confirmed the diagnosis of the fibrolamellar variant of HCC (Figures 2,3). The surrounding liver parenchyma contained changes consistent with chronic outflow obstruction including perivenular and periportal fibrosis. The segment 1 lesion was a focal nodular hyperplasia (FNH).
The patient’s post-operative course was complicated by fluid overload, pleural effusions and an increased oxygen requirement on day 2, which was managed by diuresis and bilateral percutaneous thoracentesis. Hepatic dysfunction peaked on day 2 with a total bilirubin of 2.9 mg/dL and INR of 1.8 and normalized by day 4. He remained in sinus rhythm, was restarted on warfarin on post-operative day 6, and discharged home on post-operative day 9 without other complications. He returned to full-time work after 6 weeks, and an MRI performed 8 weeks after surgery showed no signs of residual or recurrent disease (Figure 1). The patient remains free of disease at 1-year follow-up.
Discussion
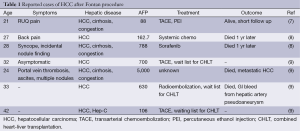
There are few reports of HCC management in patients with cirrhosis following the Fontan procedure. The existing publications describe non-surgical locoregional control using transcatheter arterial chemoembolization, radioembolization, or ablation with percutaneous ethanol injection (PEI), all with the intent of bridging to combined heart and liver transplantation (4-6) (Table 1). In one report, two patients treated with systemic chemotherapy died of metastatic disease (5). For early stage HCC, surgical resection provides definitive treatment with good long-term results, but hepatectomy is rarely performed following Fontan procedure. Our recent PubMed search failed to identify a published report of a successful liver resection. Surgery may be limited by the extent of the tumor, liver dysfunction and/or cardiac reserve in this patient population (6). Here we show that liver resection can be performed safely following the Fontan procedure in a patient with preserved cardiac and hepatic function.
Full table
A key challenge in the perioperative management of patients with post-Fontan physiology is maintaining adequate preload to ensure pulmonary blood flow (10). Intraoperative positive pressure ventilation, vasodilators, and anesthetic agents can all reduce venous return and thus cardiac output these patients. In fact, complication rates in post-Fontan patients undergoing non-cardiac procedures high; one series reported a complication rate of 31% (11). The risk of rapid blood loss makes liver resection even more challenging than other procedures in these patients. On the one hand, maintaining low CVP during hepatectomy is a key strategy to minimize operative blood loss (12), but on the other hand, higher filling pressures are required to maintain post-Fontan cardiac output. Hence, successful liver resection in these patients requires a delicate balance and tight regulation of intravascular volume. Multidisciplinary perioperative planning involving adult congenital cardiologists, anesthesiologists (cardiac and transplant), surgeons (hepatobiliary and transplant), and interventional radiologists is critical to formulate a safe pre-, intra- and post-operative assessment and management plan.
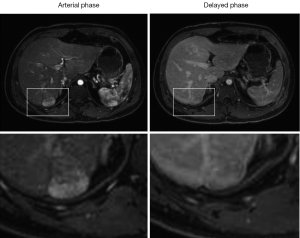
Besides preserved cardiopulmonary and hepatic function, we were able to offer surgery to our patient because of the early stage of his HCC. Currently, the role of HCC screening in post-Fontan patients remains undefined. Multiple studies have shown that evidence of liver injury and fibrosis can be detected shortly after the Fontan procedure, suggesting the possibility of pre-existing hepatic disease (13,14). Liver damage increases with the severity and duration of underlying cardiac disease, and the interval from the initial Fontan procedure (15). A retrospective analysis of 42 Fontan patients undergoing liver CT or MRI identified features consistent with cirrhosis in 19%, and hypervascular nodules in 31%, among which one had biopsy-proven fibrolamellar HCC with extensive portal venous invasion (16). Additionally, the presence of hypervascular nodules correlates with higher mean right-sided pressures and often represents FNH (17), as it has been proposed that Fontan physiology leads to chronic portal venous deprivation and secondary arterialization resulting in FNH. Given the multitude of hepatic pathologies that can be seen in this growing group of patients, more evidence is needed for the development of screening guidelines. Our patient had two lesions resected; one was an HCC and the other was FNH. Figure 4 illustrates another patient with post-Fontan circulation and a hypervascular mass with mild washout and a pseudocapsule in the delayed phase suggestive of an HCC, but biopsy of this lesion revealed an FNH. Thus, current imaging criteria for hepatic lesions based on contrast dynamics may be less reliable in the setting of post-Fontan hemodynamic alterations. One may anticipate greater use of targeted biopsies of hepatic masses in these patients, although they also carry a higher procedural risk that must be carefully weighed. The development of novel non-invasive diagnostic modalities remains a priority.
Conclusions
While post-Fontan physiology portends increased risk during liver resection due to the sensitivity of these patients to preload fluctuations, morbidity can be minimized by a multidisciplinary approach with careful coordination amongst cardiology, anesthesiology, and surgical teams. With the growing number of adult patients with post-Fontan physiology, early detection and accurate diagnosis of liver masses is of paramount importance. In patients with adequate cardiac and hepatic reserve, surgical resection of HCC can be performed safely.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Rychik J, Veldtman G, Rand E, et al. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr Cardiol 2012;33:1001-12. [PubMed]
- Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85-92. [PubMed]
- Krieger EV, Moko LE, Wu F, et al. Single ventricle anatomy is associated with increased frequency of nonalcoholic cirrhosis. Int J Cardiol 2013;167:1918-23. [PubMed]
- Izumi Y, Hiramatsu N, Itose I, et al. Juvenile hepatocellular carcinoma with congestive liver cirrhosis. J Gastroenterol 2005;40:204-8. [PubMed]
- Saliba T, Dorkhom S, O'Reilly EM, et al. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol 2010;22:889-91. [PubMed]
- Asrani SK, Warnes CA, Kamath PS. Hepatocellular carcinoma after the Fontan procedure. N Engl J Med 2013;368:1756-7. [PubMed]
- Izumi Y, Hiramatsu N, Itose I, et al. Juvenile hepatocellular carcinoma with congestive liver cirrhosis. J Gastroenterol 2005;40:204-8. [PubMed]
- Saliba T, Dorkhom S, O'Reilly EM, et al. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol 2010;22:889-91. [PubMed]
- Asrani SK, Warnes CA, Kamath PS. Hepatocellular carcinoma after the Fontan procedure. N Engl J Med 2013;368:1756-7. [PubMed]
- Eagle SS, Daves SM. The adult with Fontan physiology: systematic approach to perioperative management for noncardiac surgery. J Cardiothorac Vasc Anesth 2011;25:320-34. [PubMed]
- Rabbitts JA, Groenewald CB, Mauermann WJ, et al. Outcomes of general anesthesia for noncardiac surgery in a series of patients with Fontan palliation. Paediatr Anaesth 2013;23:180-7. [PubMed]
- Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol 2014;109:81-8. [PubMed]
- Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg 2005;129:1348-52. [PubMed]
- Schwartz MC, Sullivan L, Cohen MS, et al. Hepatic pathology may develop before the Fontan operation in children with functional single ventricle: an autopsy study. J Thorac Cardiovasc Surg 2012;143:904-9. [PubMed]
- Johnson JA, Cetta F, Graham RP, et al. Identifying predictors of hepatic disease in patients after the Fontan operation: a postmortem analysis. J Thorac Cardiovasc Surg 2013;146:140-5. [PubMed]
- Wallihan DB, Podberesky DJ. Hepatic pathology after Fontan palliation: spectrum of imaging findings. Pediatr Radiol 2013;43:330-8. [PubMed]
- Bryant T, Ahmad Z, Millward-Sadler H, et al. Arterialised hepatic nodules in the Fontan circulation: hepatico-cardiac interactions. Int J Cardiol 2011;151:268-72. [PubMed]


