Functional imaging of radiation liver injury in a liver metastasis patient: imaging and pathologic correlation
Introduction
Delivery of external beam radiation therapy (RT) to primary or metastatic liver malignancies takes into account the risk of radiation induced liver disease (RILD), a clinical syndrome characterized by right upper quadrant pain, ascites, anicteric hepatomegaly and elevated liver enzymes (especially alkaline phosphatase) that typically occurs 2-12 weeks after radiation treatment (1). The pathophysiology of classic RILD is thought to involve central vascular injury with central congestion and venous fibrosis, otherwise referred to as veno-occlusive disease (VOD) (2,3).
Radiographic changes on computed tomography (CT) imaging have been observed in the liver after RT, described as hypodense areas on portal-venous phase contrast images (4,5). However, these imaging findings after RT are characterized by morphologic and vascular changes and may not directly inform on the functional capacity of liver regions. Here we present a case demonstrating a correlation between spatial changes in liver function after focal liver RT to a liver metastasis as depicted by functional imaging with 99mTc sulfur colloid (SC) single photon emission computed tomography (SPECT/CT) and corresponding histopathologic changes.
Case report
A 69-year-old Caucasian woman was treated in 2004 for a stage I, low grade uterine carcinoma with total abdominal hysterectomy and bilateral salpingo-oophorectomy and was disease free until she presented in early 2013 with right shoulder and flank pain. Staging workup revealed a bulky mass in the right posterior hemi-diaphragm with extension into the posterior right pleural space and superior right retroperitoneum, and invasion into the liver parenchyma of segment 7. A core needle biopsy revealed a poorly differentiated carcinoma consistent with metastasis from her known primary.
Despite six cycles of carboplatin/paclitaxel chemotherapy, restaging studies five months later showed local only disease progression with interval increase in size of the large right sub-phrenic mass and increased invasion into the liver. Since the tumor appeared to invade the diaphragm and posterior chest wall, preoperative chemoradiation was recommended to reduce the risk of local recurrence. The patient received 5,000 cGy in 25 fractions of 200 cGy to the subphrenic liver mass concurrently with weekly cisplatin at 30 mg/m2. In the radiation treatment plan, a 1-cm margin was applied beyond the gross tumor volume to account for potential microscopic extension into the liver with an additional 0.5-cm margin for setup error. The 5,000 cGy prescribed dose, therefore, was delivered to a volume approximately 1.5-cm beyond the gross tumor volume. The patient subsequently underwent a right hepatectomy, partial resection of the right hemi-diaphragm, parietal pleurectomy, cholecystectomy, and diaphragm reconstruction approximately 6 weeks after completion of chemoradiation.
Gross examination of the surgical specimen revealed a tan-yellow raised mass on the anterior-superior dome of the liver and surrounding dusky discoloration secondary to radiation changes (Figure 1). The specimen was serially sectioned in the transverse plane to find a fairly circumscribed, lobulated solitary mass invading the liver parenchyma. The resection margins were free of the mass. The hepatic parenchymal congestion around the mass extended to an average distance of 1.5 cm and up to a maximum of 3.5 cm (Figure 1A). The specimen was fixed in 10% neutral buffered formalin and representative tissue sections to include the mass-liver interface were processed for routine histology. Sections were cut at 4 μm and stained with hematoxylin and eosin.
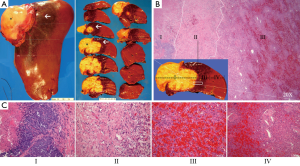
Histopathology revealed an extensively necrotic mass with focal residual poorly-differentiated carcinoma at its periphery. A zonal pattern of radiation-induced tissue changes were identified as follows: zone I demonstrated predominantly necrotic tumor cells admixed with fibrin, nuclear debris and sparse collagen strands, corresponding to the mass with small clusters of viable residual carcinoma in the periphery (Figure 1B,C, zone I). Immediately adjacent to the necrotic mass, denser bundles of collagen with chronic inflammatory cells, macrophages and increased vascular structures were identified (Figure 1B,C, zone II). The next zone demonstrated viable but atrophied and distorted hepatic cords with sinusoidal dilatation, congestion and intracellular pigment accumulation (Figure 1B,C, zone III). Distal to the congested zone, normal hepatic architecture was observed (Figure 1B,C, zone IV).
SC SPECT/CT was performed one month post-chemoradiation (2 weeks prior to surgery) to spatially characterize functional liver volume for preoperative planning and showed a region of SC photopenia that extended beyond the boundaries of the gross tumor volume (Figure 2). Furthermore, differential SC uptake appeared to correlate with varying doses of radiation delivered when registered to her radiation treatment planning CT scan. These SC uptake changes were correlated with gross pathologic changes seen in the irradiated liver specimen (Figure 3). While some degree of geometric distortion was expected from the surgical resection, there was a clear correlation between the location and size of the irradiated sections of the liver (Figure 3A) and the margin of absent SC uptake surrounding the gross tumor (Figure 3B). At last follow-up, the patient was alive and disease-free 9 months after her surgery.
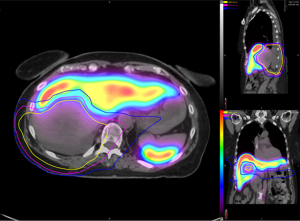
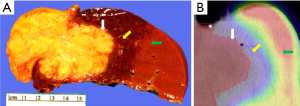
Discussion
Accurate assessment of liver function is an essential component in the management of primary and metastatic liver cancers, particularly in patients with compromised liver function planned to receive potentially hepatotoxic liver-directed therapies such as RT. Currently, evaluation of liver function routinely incorporates conventional laboratory blood tests (e.g., bilirubin, albumin, coagulation factors) and clinical variables (e.g., presence of ascites or hepatic encephalopathy) that indirectly measure liver function. These assessment tools are susceptible to subjectivity and confounding factors, may be unreliable in predicting hepatotoxicity after liver-directed treatment (6), and do not take into account potential spatial heterogeneity of liver function that may exist within the liver. Conventional imaging modalities such as CT only portray anatomic or morphologic features, but not the functional characteristics of the liver. A non-invasive, quantitative functional imaging tool that more accurately depicts global and regional liver function, therefore, would be clinically useful. In this report, we present the first clinical case, to our knowledge, of imaging-pathology correlation between radiation changes depicted on functional SPECT/CT imaging and corresponding histopathology changes on surgical pathology. We also have shown a radiation dose response correlation between delivered radiation dose and differential uptake of SC in the liver.
SC SPECT/CT was used as the imaging modality to image liver function for several reasons. It is a well-established FDA-approved diagnostic imaging modality for the evaluation of hepatic function. SC is primarily taken up and cleared by the reticuloendothelial (Kupffer) cells that line the sinusoids of the liver and serve as supporting cells to hepatocytes, as well as in the spleen and bone marrow. It has been established that a close correlation exists between the function of hepatocytes and Kupffer cells (7,8). SC function, therefore, can be used as a surrogate for hepatic function. Furthermore, multiple studies have demonstrated that SC distribution within the liver correlates well with histologic fibrosis in liver explants, presence and severity of cirrhosis, and long-term clinical outcomes of hepatic failure-related mortality (9-11).
Our pathologic findings are consistent with recently described findings from Olsen et al. (5). These investigators examined radiographic and pathologic changes in the liver after the delivery of high doses of hypofractionated stereotactic body RT and described similar zonal radiation injury patterns as in our study. In their study, the 3,000 cGy dose (delivered over 3 fractions, which is radiobiologically equivalent to approximately 7,800 cGy delivered in 200 cGy per fraction) roughly corresponded to the VOD zonal injury (type III). However, in our study, we found that the 4,500 cGy dose (delivered in conventional 200 cGy per fraction with concurrent chemotherapy) to be spatially consistent with the VOD type III zonal injury on histopathology, as well as correlated with abrogated SC uptake on SPECT/CT imaging. It is unclear if this discrepancy is due to different fractionation schemes and/or differences in imaging modality. It is noteworthy that the imaging findings in the Olsen et al. study were based on decreased perfusion changes in the irradiated region, which does not necessarily imply that the liver parenchyma in that region is impaired or injured.
In contrast, our case report illustrates an example of how spatial changes depicted on SC SPECT/CT imaging (that reflect changes in the regional functional capacity of the liver) are correlated with delivered radiation dose as well as histopathologic changes of known radiation liver injury. This supports additional investigation into the use of SC SPECT/CT as a non-invasive method to assess liver function for liver-directed treatments. This tool may also allow physicians to deliver safer treatments to liver cancer patients, particularly in those with compromised liver function by: (I) more accurately assessing pre-treatment global liver function, which would assist in determining which patients should receive more aggressive treatments; and (II) characterizing spatial heterogeneity of function within the liver, which would allow for personalized treatment approaches such as radiation functional avoidance planning (redistributing radiation dose away from highly functioning regions of liver).
Acknowledgements
Disclosure: The authors declared no conflict of interest.
References
- Ingold JA, Reed GB, Kaplan HS, et al. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med 1965;93:200-8. [PubMed]
- Reed GB Jr, Cox AJ Jr. The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol 1966;48:597-611. [PubMed]
- Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995;31:1237-48. [PubMed]
- Herfarth KK, Hof H, Bahner ML, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys 2003;57:444-51. [PubMed]
- Olsen CC, Welsh J, Kavanagh BD, et al. Microscopic and macroscopic tumor and parenchymal effects of liver stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:1414-24. [PubMed]
- Garcea G, Ong SL, Maddern GJ. Predicting liver failure following major hepatectomy. Dig Liver Dis 2009;41:798-806. [PubMed]
- Hoefs J, Chang K, Wang F, et al. Perfused Kupffer cell mass. Correlation with histology and severity of chronic liver disease. Dig Dis Sci 1995;40:552-60. [PubMed]
- Horisawa M, Goldstein G, Waxman A, et al. The abnormal hepatic scan of chronic liver disease: its relationship to hepatic hemodynamics and colloid extraction. Gastroenterology 1976;71:210-3. [PubMed]
- Hoefs JC, Wang F, Kanel G. Functional measurement of nonfibrotic hepatic mass in cirrhotic patients. Am J Gastroenterol 1997;92:2054-8. [PubMed]
- Zuckerman E, Slobodin G, Sabo E, et al. Quantitative liver-spleen scan using single photon emission computerized tomography (SPECT) for assessment of hepatic function in cirrhotic patients. J Hepatol 2003;39:326-32. [PubMed]
- Everson GT, Shiffman ML, Hoefs JC, et al. Quantitative liver function tests improve the prediction of clinical outcomes in chronic hepatitis C: results from the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis Trial. Hepatology 2012;55:1019-29. [PubMed]


