Emergency surgery for perforated gastric malignancy: An institution’s experience and review of the literature
Digestive Disease Centre, Department of General Surgery, Tan Tock Seng Hospital, Singapore
|
Original Article
Emergency surgery for perforated gastric malignancy: An institution’s experience and review of the literature
Digestive Disease Centre, Department of General Surgery, Tan Tock Seng Hospital, Singapore
|
|
Abstract
Background: The aim was to evaluate the outcome of patients who underwent surgery for perforated gastric malignancies.
Methods:A review of all patients who underwent surgery for perforated gastric malignancy was performed.
Results: Twelve patients (nine gastric adenocarcinoma and three B-cell lymphoma) formed the study group. Ten (83.3%) had subtotal gastrectomy performed, while two (16.7%) underwent total gastrectomy. All eight patients with adenocarcinoma who survived the initial operation fared poorly. The two patients with lymphoma who survived the surgery underwent subsequent chemotherapy has no disease recurrence currently.
Conclusion: Surgery in perforated gastric malignancy is fraught with numerous challenges.
Key words
emergency, surgery, perforation, treatment outcome, malignancy
J Gastrointest Oncol 2011; 2: 13-18. DOI: 10.3978/j.issn.2078-6891.2011.001
|
|
Introduction
Perforated gastric malignancy is a surgical emergency
fraught with numerous challenges. Although the diagnosis
of a perforation can be easily achieved, the differentiation
between a malignant and benign aetiology remains elusive
(1,2). This has serious implications as it often determines
the extent of the operation.
The aims of surgery in these patients are two-fold: to
manage the peritoneal contamination and the underlying
malignancy. While managing the peritoneal contamination
could be easily handled, the ideal operation in treating
the malignancy is perplexing as it is dependent on various
factors such as the haemodynamic stability of the patient,
the surgical expertise and the stage of the malignancy
(3-6). To perform a complete oncologic resection may be
too hazardous for the patient, whereas a limited procedure
could significant impact the long-term survival of these
patients.
The short-term outcome in these patients is often poor
due to the septic complications from the perforation and
may be further contributed by any concurrent resection
surgery (3-6). Moreover, the long term outcome in these
patients may be unfavourable due to the likely advanced
stage of the gastric malignancy and the possibility of tumour
seeding of the peritoneal cavity through the perforation
(3-6).
Due to the relative rarity of this topic being discussed
in the literature, this review was performed to evaluate the
presentation and the short- as well as the long-term outcome
of patients who underwent urgent surgery for perforated
gastric malignancies.
|
|
Methods
Study population
Tan Tock Seng Hospital is a 1400 bed hospital, the second
largest in Singapore and provides secondary and tertiary
medical care for about 1.5 million people. A retrospective
review of all patients who underwent emergency surgery
for perforated gastric malignancy from October 2003 to
March 2009 was performed. Patients were identified from
the hospital’s diagnostic index and operating records. All
malignancies were confirmed upon histological evaluation.
The data collected included age , gender, ASA
(American Society of Anesthesiologists) score and comorbid
conditions. In addition, operative findings and inter ventions, length of surgery, peri-operative
complications, mortality and length of hospital stay were
also documented.
Prior to the surgery, fluid resuscitation, nasogastric tube,
parenteral antibiotics and proton pump inhibitor would be
administered to every patient. Intra-operatively, all patients
underwent copious lavage of the peritoneum and mass
closure of the fascia. The extent of resection was determined
by the primary surgeon intra-operatively and all cases were
operated by a surgeon of at least Consultant grade.
Disease recurrence was confirmed through radiological
and/or pathological evaluation, while the overall survival
duration was documented from the date of surgery until the
date of death. All gastric cancers were staged according to
the guidelines of the American Joint Committee of Cancer
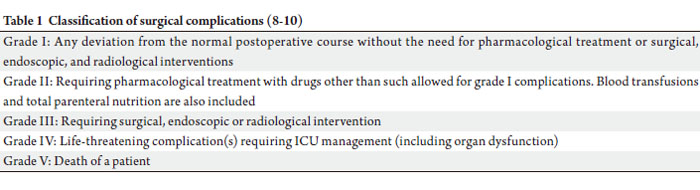
(AJCC) (7). The grades of complications (GOC) were in
concordance to the classification proposed by Clavien and
group (8-10) (Table 1).
 |
|
Results
During the study period, twelve patients (n = 8, 66.7%
males) underwent surgery for perforated gastric cancer.
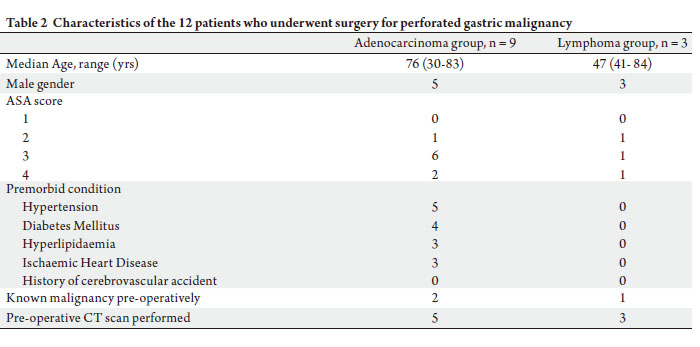
Gastric adenocarcinoma and B-cell lymphoma were
responsible for the perforation in nine (75.0%) and three
(25.0%) patients respectively. Three had their gastric
malignancy diagnosed prior. The median age of the study
group was 75 (30~84) years, with the majority (n = 10,
83.3%) having an ASA score of 3 or 4.
All patients presented with severe abdominal pain.
Pneumoperitoneum on erect chest radiographs was seen
in five (41.7%) patients while emergency confirmatory
computed tomographic (CT) scans were performed in the
rest. Majority (n = 9, 75.0%) of patients underwent surgery
within 24 hours of presentation. Table 2 highlights the
various characteristics of the study group.
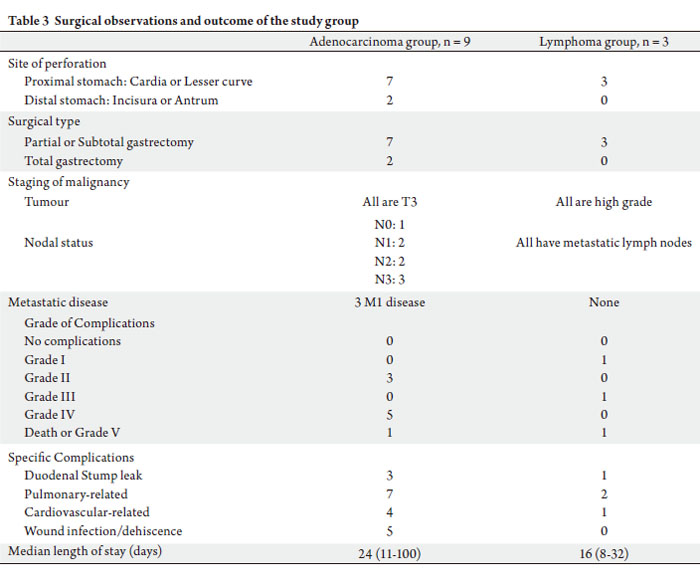
Intra-operatively, seven (59.3%) patients have severe
peritoneal contamination. Ten (83.3%) had partial
or subtotal gastrectomy performed with Bilroth II anastomosis, while the remaining two (16.7%) underwent
total gastrectomy with a resulting Roux-en-Y anastomosis.
Two patients died from septic complications contributed
by pneumonia and intra-abdominal sepsis, one of whom
had a duodenal stump leak which necessitated a subsequent
laparotomy, drainage of the intra-abdominal collections and
repair of duodenal stump dehiscence. The remaining ten
patients were discharged well after a median length of stay
of 16 (range: 8~100) days. Table 3 illustrates the surgical
observations, procedure and outcome.sification proposed by Clavien and
group (8-10) (Table 1).
Apart from the duodenal stump leak above, three other
patients had duodenal stump leaks that were managed
conservatively. Almost all the patients had either pulmonary
or cardiovascular complications post-operatively.
Adenocarcinoma
Nine patients had adenocarcinoma. All had T3 disease and
the only patient with N0 disease was one of the fatalities,
the rest of the patients all had involved lymph nodes. Three
patients had metastatic disease diagnosed concurrently with
peritoneal (n = 3) and liver (n = 1) involvement.
Eight patients survived the initial operation. In the
three patients with metastatic disease, one foreign patient
defaulted follow up and went back to his home country. The
other two passed away from their advanced disease at three
and ten months post-operatively, respectively. Both did not
undergo any palliative chemo- or radio-therapy.
In the remaining five patients, one defaulted three
months after the surgery. Two other patients had disease
recurrence in the peritoneum causing intestinal obstruction
within eight months of the initial surgery. Both perished
within a few months subsequent to that. Both did not
undergo any adjuvant chemo- or radio-therapy.
Only two patients in this group underwent adjuvant
chemo- and radio-therapy in whom one had hepatic and
pulmonary metastases ten months post-operatively and
passed away seventeen months after. The other patient
had spinal metastases diagnosed sixteen months after the
surgery. He declined further chemo and radio-therapy and defaulted follow up subsequently.
Lymphoma
Two patients survived the initial surgery and both
underwent subsequent chemotherapy and are still on strict
surveillance under the medical oncologist. Currently, both
are well with no evidence of disease recurrence.
  |
|
Discussion
Even though the incidence of malignant gastric perforation
remains low, the consequences are considerable (1,2). Our
series affirmed the dismal peri-operative outcome following
surgery in these patients. Two patients (16.7%) died with
another six (50.0%) having severe complications (GOC
III and IV). Similar to other reports, the majority of these
complications are attributed to cardio-respiratory and septic
causes (11-15).
Though malignancy has been quoted as an independent
factor predicting worse outcome in gastric perforation,
other more commonly associated adverse factors would
include pre-operative shock, poor pre-morbid condition,
advanced age, delayed presentation and resection surgery
(11-16). Over the years, several scoring systems have been
advocated in the prognostication of patients with gastric
perforation, with Boey score being commonly adopted and
validated in several reports (15,16).
Boey score utilized three independent factors of
concomitant severe medical illness, pre-operative shock
and long-standing perforation with predicted mortality
rate of over 80% if all three factors are present. However,
one of its main criticisms has been its inability to consider
other physiological and intraoperative parameters. This
has resulted in the numerous other scoring systems such
as the Mannheim peritonitis Index (MPI), ASA score and
APACHE II being adopted, each with its advantages and
limitations. Suffice to say, the outcome in these patients are
dependent on a combination of patient, disease and surgeon
factors.
To make matter worse, in the absence of a known
pre-operative gastric malignancy, it may be difficult
to accurately diagnose the presence of malignancy
in any gastric perforation (1,2). Mistaking a benign
ulcer perforation as malignant is not impossible given
the significant surrounding induration and enlarged
inf lammatory lymph nodes. This may subject the patient
to an unnecessary extensive and resection surgery with its
numerous associated complications (1-6,17). Some of the
clues suggestive of a malignant perforation would include
advanced age, size of ulcer > 6cm and size of perforation
> 0.5cm, raised white cell counts and longer duration of symptoms (1). The importance of frozen section intraoperatively
has been emphasised to clinch the diagnosis
but it may not be always available and false negative is also
possible. In our series, frozen section was not performed
in any patients as it was either not available or deemed
not necessary by the primary surgeon because of the size
of the ulcer and perforation, or if the malignancy was
clinically suspected or already diagnosed. These would have
supported the decision for gastrectomy regardless of the
outcome of frozen section.
Even when the malignant perforation could be accurately
diagnosed, the surgical procedures of choice in these
patients are often dependent on various factors. These
would include the presence of metastatic disease, expertise
of the surgeon in performing an oncologic resection, the
degree of contamination and perhaps most importantly, the
intra-operative haemodynamic status of the patient.
At one stage, malignant gastric perforation has been
deemed as terminal disease due to the associated peritoneal
dissemination and early recurrences (18-20). This had
led to the practice of simple closure of the perforation
(21,22). However, this technique has been associated with
unacceptable peri-operative complications and hence
abandoned. Perhaps this should only be considered when
the patient is extremely haemodynamically unstable to
withstand any resection.
Over the years, the morbidity following emergency
gastrectomy has been improving due to improving surgical
technique and advancement in critical care (23). This
has become the preferred surgical option in patients with
malignant gastric perforation. Not only is it able to tackle
the perforation, it can also remove the underlying pathology.
However, the extent of radical oncologic surgery is perhaps
dependent on the aforementioned factors. While it may be
dangerous to embark on a major radical oncologic resection,
the implications of a limited procedure may seriously
impact the long term survival in patients with potentially
curable gastric malignancy. This had led to the adoption
of a two-stage procedure in handling this perplexing
situation (3,24). While the first stage aimed to tackle the
peritoneal contamination and the gastrectomy, the second
procedure would be performed at a later date to ensure
adequate lymph node clearance. However, the problems of
such a staged procedure would include the significant postoperative
adhesions from the first surgery, and also the
fitness of the patient to withstand another extensive surgery.
In addition, this could delay the commencement of any
chemo- and radio-therapy, especially if any complications
were encountered.
Recent data have disproved the notion that gastric
perforation often resulted in increased risks of recurrences and peritoneal disease. The long term survival of patients
with per forated gastric adenocarcinoma is actually
comparable to patients performed electively (3-6). The
only factor determining long term survival is the stage
of the malignancy. As seen in our series, the majority of
our patients had very advanced disease on diagnosis and
fared badly subsequently with almost all the patients
developing disease recurrences. Though several of our
patients developed peritoneal disease subsequently, it
could be related to the advanced staging and progression
of the primary malignancy rather than contributed by the
perforation. Unfortunately, large series is not available in
the literature to shed more light into this.
The role of surgery in gastric lymphoma has been
addressed by numerous reports and should only be
performed as a primary radical treatment, palliative
procedure or when emergency complications such as
massive bleeding or perforation are encountered (25-28).
The implications of the gastric perforation in the long term
survival of these patients appear minimal with no reports
of associated recurrence reported. The most important
factor determining the long term survival is again the stage
of the lymphoma. None of our patients had any systemic
or peritoneal recurrence and both are currently well upon
completion of their chemotherapy.
|
|
Conclusion
Surgery in perforated gastric malignancy is fraught with
numerous challenges. Short-term outcome is dismal and
is dependent on the various patient and disease factors.
Long-term survival in these patients is dependent on the
underlying stage of the malignancy.
|
|
References
Cite this article as:
Tan K, Quek T, Wong N, Li K, Lim K. Emergency surgery for perforated gastric malignancy: An institution’s experience and review of the literature. J Gastrointest Oncol. 2011;2(1):13-18. DOI:10.3978/j.issn.2078-6891.2011.001
|