Human papillomavirus tumor infection in esophageal squamous cell carcinoma
Introduction
Human papillomavirus (HPV) is a circular, double-stranded DNA virus with established oncogenic potential in the setting of multiple malignancies, most prominently squamous cell carcinoma of the cervix (1). The established role of HPV in squamous cell cancers of other sites, including the anus and the oropharynx, has resulted in a growing literature aiming to assess the biological and clinical roles of HPV tumor infection in a diversity of cancers. Evidence dating back over three decades has indicated an association between HPV infection and esophageal squamous cell carcinoma (ESCC) (2,3). Recent meta-analyses have assessed the incidence and etiological role of HPV-ESCC tumor infection. This review aims to provide clinicians with a summary of the current HPV-ESCC literature and clinical recommendations regarding HPV infection in this disease.
HPV-ESCC infection rates
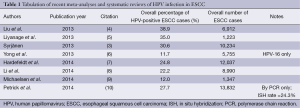
Perhaps the most well-studied and yet controversial issue with regard to HPV infection in the setting of ESCC is the overall rate of HPV infection in these tumors. The question of whether, or to what extent, HPV infection occurs in ESCC has been the subject of ongoing debate. Dozens of studies have attempted to address this question using a variety of techniques, controls, and patient populations, as will be discussed below. More recently, a number of meta-analyses and systematic reviews have been published, attempting to address this question. Table 1 summarizes these, providing an overall rate of HPV-ESCC infection identified across studies. While these meta-analyses draw upon much of the same primary literature, each utilizes differing criteria with regard to the type of studies included. As illustrated in Table 1, worldwide HPV-ESCC infection rates range from 11.7% to 38.9%. Of note, one of these analyses provides separate data for high-risk HPV types 16 and 18 (HPV-16 and HPV-18); these authors report an 11.7% infection rate for HPV-16, and a 1.8% infection rate for HPV-18, but do not provide pooled data for both high-risk HPV types (6). Issues pertaining to strain-specific HPV identification will be discussed further below.
Full table
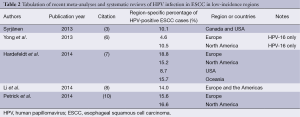
The wide range of HPV-ESCC infection rates reflects the variability in the primary literature, where even large-scale case-control studies have reported infection rates between 0% and 78% (11,12). While many reasons for this variability have been postulated, geographic variation remains among the strongest predictors for observed disparities in infection rates (3). There are wide geographic differences in the overall incidence of ESCC, with high-incidence countries (e.g., China and Iran) reporting one-hundred-fold higher rates of ESCC compared with low-incidence countries (e.g., Australia and the United States) (13). Low-incidence countries have ESCC rates of approximately 2.5 per 100,000, whereas high-incidence countries have rates as high as 250 per 100,000 (13,14). Interestingly, in high-ESCC-incidence countries, the HPV tumor infection rate is also significantly higher relative to low-ESCC-incidence countries (3). Multiple meta-analyses have demonstrated this difference, with reported rates of HPV-ESCC tumor infection in China on the order of 32.8-63.6% (3-5,7). Low-incidence regions, including North America, have substantially lower reported rates of HPV-ESCC infection (Table 2). For North America alone, rates range between 8.7% and 16.6% (3,6,7,10).
Full table
Given these geographic differences in HPV-ESCC infection rates, an important caveat is that worldwide HPV prevalence estimates are biased by the number of cases and studies from specific regions. In most meta-analyses, investigators report the majority of studies used in their analyses are from Asia alone, a high-risk region (3,4,6,7,10). This publication bias toward high-incidence regions complicates interpretation of the overall reported HPV-ESCC prevalence rates in Table 1. We therefore included Table 2 to facilitate a geographically-specific interpretation of the reported HPV-ESCC infection rates primarily in a Western population. One meta-analysis, for instance, calculated an overall HPV prevalence of 30.6%, but a region-specific infection rate of 10.1% for Canada and the United States (3). The differences between reported worldwide and low-incidence-region HPV-ESCC rates, even within the same meta-analysis, demonstrates the degree to which geographic publication bias affects the reported overall infection rates. Why this asymmetric geographic distribution exists, however, remains largely unanswered. Some evidence has suggested that environmental exposures and nutritional deficiencies might provide an etiological basis for higher ESCC incidence in the developing world (3,13).
Methodological considerations
Despite evidence that geography accounts for much of the variability observed in the primary literature measuring HPV-ESCC infection rates, another area of concern relates to the different methods used to evaluate HPV status in patient samples (3,7). Historically, methods have included histology, Southern blotting, dot blot hybridization, HPV L1 protein serology, in situ hybridization (ISH), and polymerase chain reaction (PCR), with most contemporary studies utilizing ISH or PCR. Comparing these two, PCR has high specificity but also a high false positive rate, whereas ISH has high specificity but low sensitivity (15). Several reviews have proposed that variability in reported HPV-ESCC infection rates may be at least partially explained by HPV detection method (3,13,16,17). One recent systemic review found the overall HPV-ESCC prevalence varied significantly between less-used methods, from 17.6% for the now-outdated Southern blot technique to 32.2% for HPV L1 serology (10). However, as shown in Table 1, this review found that the two most commonly-used methods, PCR and ISH, demonstrate similar overall HPV-ESCC rates of 27.7% and 24.3%, respectively, with no statistically significant difference between the PCR and ISH HPV prevalence rates (10). Along these lines, other meta-analyses have concluded that detection method does not account for the variability in reported HPV-ESCC infection rates, as this variability persists even when examining studies all utilizing the same detection method (3,5,10). Collectively, these analyses suggest it is unlikely that choice of detection method accounts for a significant portion of the observed HPV prevalence variability.
Since variability persists within studies using the same detection method, it is worthwhile to examine any potential intra-method technical factors that may influence reported HPV prevalence rates. Looking at the most prevalent detection technique, PCR, one methodological issue is the lack of a ‘gold standard’ primer set for use in this approach (6). In PCR-based studies, investigators have utilized both type-specific and broad-spectrum primers; type-specific primers tend to amplify short segments of DNA, making these primers more sensitive than broad-spectrum primers which amplify longer DNA stretches (6). Consistent with this, one meta-analysis showed that HPV-16/18 prevalence is higher when type-specific primers were used (versus broad-spectrum primers) (6). Even when controlling for these two broad categories of primers, issues remain with the PCR detection method, including which specific primer set is used in a given study, as well as rapidly-advancing PCR technologies.
The discussion regarding PCR primers leads to another concern in these studies: the selection of HPV strains to be probed. Over 100 HPV strains have been identified, with only a fraction of these implicated in the development of cancer, termed high-risk strains. It is worth noting that the high-risk and low-risk distinctions were broadly developed based on literature from cervical carcinoma. These designations were validated by data from oropharyngeal and anal cancers, where the vast majority of HPV-positive squamous cell carcinoma lesions are caused by the previously-identified high-risk HPV genotypes (notably HPV-16, -18, and -33) (1,18-20). The variation between studies as to which HPV strains are interrogated results in potential selection bias, affecting estimated HPV-ESCC prevalence rates. There is also an assumption in the literature that the same high-risk genotypes identified in other settings should also be considered high risk in ESCC. If HPV is an etiologic agent in the setting of ESCC, it may have a similar or dissimilar strain profile to that of other disease sites; this phenomenon has been observed previously, where the HPV strain profile differs based on cancer site (18-20). In esophageal cancer, one meta-analysis described a significant association between HPV-16 and ESCC, but not HPV-18 (6). As shown in Table 1, this analysis reported an overall HPV-16 prevalence rate of 11.7%, vs. 1.8% for HPV-18 (6). This is consistent with other systematic reviews showing HPV-16 as the most commonly identified strain in HPV-ESCC infection across multiple methods (10). Regarding HPV-18, however, there is some disagreement within the literature; while the analysis above cites no significant HPV-18 association with ESCC, other studies have identified HPV-18 as the second most prevalent HPV-ESCC genotype (21-24). The HPV strain profile for ESCC remains elusive, with some reviews suggesting a heavily HPV-16-predominant profile as in the case of oropharyngeal lesions (6,19). However, several studies examining multiple HPV genotypes across ESCC samples found virtually no incidence of HPV-ESCC infection, irrespective of genotype (25,26). Future studies may benefit from standardizing detection method and expanding the selection of HPV strains being probed.
Other methodological and study-design considerations complicate data interpretation. In many studies, tissue samples derived from endoscopic biopsies may lead to sampling error or insufficient tissue for testing (27). Tissue stability and quality may also be affected by overall DNA concentration, solution pH, use and duration of formalin fixation, temperature, and type of tissue storage (28). Conflicting reports have suggested that formalin-embedding might enhance or reduce rates of HPV detection in ESCC samples (6,28,29). Several groups have also raised concern over sample quality control and potential contamination, as HPV fomites can be found on medical surfaces and are often resistant to commonly used detergents. Potential contamination of samples would complicate any HPV-ESCC analysis (10). Of note, among the studies with the most rigorous methodological controls, one large series from a particularly high-incidence region within China found essentially no HPV-ESCC correlation (26). Regarding study-design, case-control methods have traditionally been used to assess potential links between cancer and environmental factors. One analysis drawing upon case-control studies alone found a three-fold increase in the risk of ESCC with HPV infection (5). Focusing on case-control literature is likely of particular importance given that HPV detection rates in normal esophagus have been reported as high as 58.9% in some studies, and as low as 0% in others (11,23). Lastly, most series have relatively small sample sizes, likely making most underpowered to answer questions of HPV-ESCC prevalence (10).
Etiological role of HPV in ESCC
Given the diversity of HPV genotypes and their relative contributions to oncogenesis in established clinical paradigms such as cervical cancer and head and neck cancers (HNC), it is clear that measuring the oncogenic activity of HPV infection is critical to understanding the etiological role of HPV-ESCC infection (1). To this end, the p16/INK4A (p16) biomarker has been used as a measure of HPV oncoprotein activity. HPV E7 protein inactivates retinoblastoma protein, consequently releasing E2F transcription factors, which then promote both cell-cycle progression and p16 expression (30). Immunohistochemical evidence of p16 overexpression has been widely used as a correlate for HPV oncogenic activity in cervical cancer and dysplasia (31,32). In oropharyngeal lesions, over 90% of high-risk HPV-positive lesions also overexpress p16 (19). Although 10-20% of p16-positive HNC cases are HPV-negative, it is noteworthy that p16 overexpression is a positive prognostic factor, irrespective of HPV status (20,33-37). Together, these data highlight the role of p16 overexpression in other sites as a functional correlate for HPV-mediated oncogenesis (20,31).
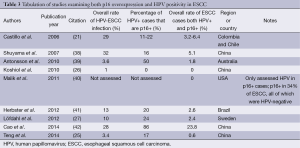
Although there are many potential pitfalls in determining rates of HPV tumor infection, including issues of geography, HPV detection method, and others, none of these address the issue of biological significance of HPV-ESCC infection. Assessing functional correlates for HPV oncoactivity is particularly important in the context of multiple HPV-ESCC case-control studies showing HPV infection rates of greater than 50% in the normal esophagus (12,23). Recent meta-analyses have not evaluated p16 overexpression or any other functional correlate for HPV oncoactivity to characterize the potential etiological role of HPV infection in ESCC (5,8). The available data, as a whole, are markedly different from the oropharyngeal and cervical carcinoma literature. Studies of HPV infection in ESCC have shown little to no agreement between p16 overexpression and HPV-positivity. As described previously, over 90% of HPV-positive oropharyngeal cancers are p16 overexpressing (19), but the association between p16 and HPV in ESCC remains limited (Table 3). With one exception (42) the data presented in Table 3 suggest a low rate of double-positive (HPV-positive and p16-overexpressing) ESCC lesions, around or below 5% of all cases (21,25-27,38-41). These conclusions are supported by a recent systematic review that demonstrated p16 overexpression to be an unreliable marker for HPV status in ESCC, with an odds ratio of HPV-positivity in a p16-overexpressing ESCC lesion of 1.07 [95% confidence interval (CI), 0.70-1.62] (9).
Full table
These data lead to questions regarding the etiological significance of HPV infection in ESCC. Despite studies from other sites confirming the role of p16 overexpression as a reliable marker for HPV oncoactivity, might HPV still be playing a carcinogenic role in ESCC without induction of p16 overexpression? Several hypotheses have been proposed suggesting this may be the case. One hypothesis states that ESCC carcinogenesis may involve a high rate of p16 promoter methylation, thereby inhibiting p16 expression even in the setting of oncogenic HPV infection. One study supporting this hypothesis found over 70% of ESCC cases with loss of p16 expression secondary to promoter methylation, as compared to another study demonstrating p16 loss in only 20% of oropharyngeal lesions (43,44). Nevertheless, a lack of correlation between p16-overexpression and HPV oncoactivity in ESCC would be unusual given that p16 is used clinically as a proxy for HPV carcinogenic activity in multiple other sites (20,31). One systematic review, drawing upon multiple case-control studies, attempted to address the question of HPV oncoactivity in ESCC using another method. These investigators utilized HPV-16 and -18 E6 and E7 protein serology to assess the functional association between ESCC and HPV (45). High-risk HPV E6 and E7 protein serology is a highly-specific marker for HPV-driven squamous cell carcinomas of multiple sites, including the cervix and oropharynx (45-49). Synthesizing their findings, the investigators found a limited association between HPV and ESCC based on serology. This led them to conclude that HPV may contribute to the etiology of a small subset of ESCC, but is unlikely to represent an important oncogenic risk factor (45).
Collectively, p16-overexpression and HPV serological data appear to indicate that despite reported rates of HPV infection in ESCC, HPV may not play a significant oncogenic role in ESCC. This is complicated by geographic variation highlighted previously, wherein regions with the highest rates of ESCC also have the highest rates of HPV prevalence and HPV-ESCC infection. The geographic picture correlating HPV with ESCC may suggest a causal link between the two. However, this geographic correlation is taken with caution as studies even from the same geographic regions report tremendous variability in HPV-ESCC infection rates (12,26). Contemporary literature appears to indicate that HPV likely does not play a significant etiological role in the vast majority of ESCC cases, particularly in low-incidence regions.
Clinical relevance and recommendations
While HPV does not appear to be a significant etiologic agent in ESCC, is it possible HPV infection might offer prognostic significance in esophageal cancer? Several investigators have evaluated the prognostic role of HPV-ESCC infection. However, in aggregate, these data are conflicting (Table 4). In one series, HPV infection in ESCC was shown to be a poor prognostic indicator (50). In contrast, another recent series showed improved overall and disease-free survival in ESCC patients with HPV-positive tumors (42). Several other studies still have failed to show any significant association between HPV infection and patient survival (Table 4) (22,39,51). This is in contrast to oropharyngeal lesions, where HPV-positivity has been consistently shown to be a strong positive prognostic factor in patient outcomes (19,34,52,53). The outcomes data for HPV in ESCC, however, remains both limited and conflicting, precluding definitive interpretation.

Full table
Another clinical question is whether a specific subset of ESCC patients may be more likely to have HPV-positive lesions. Preliminary evidence suggests HPV-positive ESCC may be more common among patients that are less than 55 years old, male, of higher BMI, or smokers (39,54). Other studies have examined whether location of the primary ESCC lesion within the esophagus predicts HPV prevalence rates. The notion that upper esophageal lesions may have higher incidence of HPV infection is grounded in data demonstrating higher risk of HPV-positive ESCC in patients with prior or concurrent HPV-positive HNC (55). However, studies investigating differential HPV rates in proximal versus distal esophageal lesions have concluded that no association exists between primary esophageal tumor location and HPV prevalence (27,56).
There have been concerns about whether clinicians should test for HPV in patients with ESCC. Based on the evidence described above, it currently appears unlikely that HPV is clinically or etiologically relevant for ESCC. We currently do not recommend that ESCC patients be tested for HPV tumor infection outside the context of a research study. At present, there is no clear indication to change clinical practice or treatment strategy for ESCC lesions based on HPV status.
Conclusions
The role of HPV in the setting of ESCC remains ill-defined. Worldwide, HPV-ESCC tumor infection rates are highly variable, such that HPV prevalence correlates strongly with high-ESCC-incidence regions. In countries such as the United States, HPV-ESCC infection rates are low, on the order of 5-15%. Geographic differences and methodological heterogeneity complicate interpretation of the current studies, leading to variable conclusions. In particular, future investigations should rely upon case-control paradigms, given the high rates of HPV infection observed in the normal esophagus. Regardless of HPV-ESCC infection rates, p16 overexpression and HPV serological data do not presently support a definitive etiological role for HPV in ESCC. Similarly, clinical data do not suggest a prognostic role for HPV infection in these lesions. Although this issue warrants further investigation, there is currently no basis to change the clinical approach to ESCC patients.
Acknowledgements
The authors thank Dr. Jill Koshiol, PhD, for her comments and assistance with this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Flint SJ, Enquist LW, Racaniello VR, et al. eds. Principles of Virology. Third Edition. Washington DC: ASM Press, 2009.
- Syrjänen KJ. Histological changes identical to those of condylomatous lesions found in esophageal squamous cell carcinomas. Arch Geschwulstforsch 1982;52:283-92. [PubMed]
- Syrjänen K. Geographic origin is a significant determinant of human papillomavirus prevalence in oesophageal squamous cell carcinoma: systematic review and meta-analysis. Scand J Infect Dis 2013;45:1-18. [PubMed]
- Liu H, Li J, Diao M, et al. Statistical analysis of human papillomavirus in a subset of upper aerodigestive tract tumors. J Med Virol 2013;85:1775-85. [PubMed]
- Liyanage SS, Rahman B, Ridda I, et al. The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis. PLoS One 2013;8:e69238. [PubMed]
- Yong F, Xudong N, Lijie T. Human papillomavirus types 16 and 18 in esophagus squamous cell carcinoma: a meta-analysis. Ann Epidemiol 2013;23:726-34. [PubMed]
- Hardefeldt HA, Cox MR, Eslick GD. Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis. Epidemiol Infect 2014;142:1119-37. [PubMed]
- Li X, Gao C, Yang Y, et al. Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment Pharmacol Ther 2014;39:270-81. [PubMed]
- Michaelsen SH, Larsen CG, von Buchwald C. Human papillomavirus shows highly variable prevalence in esophageal squamous cell carcinoma and no significant correlation to p16INK4a overexpression: a systematic review. J Thorac Oncol 2014;9:865-71. [PubMed]
- Petrick JL, Wyss AB, Butler AM, et al. Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases: systematic review and meta-analysis. Br J Cancer 2014;110:2369-77. [PubMed]
- Koh JS, Lee SS, Baek HJ, et al. No association of high-risk human papillomavirus with esophageal squamous cell carcinomas among Koreans, as determined by polymerase chain reaction. Dis Esophagus 2008;21:114-7. [PubMed]
- Cao B, Tian X, Li Y, et al. LMP7/TAP2 gene polymorphisms and HPV infection in esophageal carcinoma patients from a high incidence area in China. Carcinogenesis 2005;26:1280-4. [PubMed]
- Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol 2002;55:721-8. [PubMed]
- Chang F, Syrjänen S, Wang L, et al. Infectious agents in the etiology of esophageal cancer. Gastroenterology 1992;103:1336-48. [PubMed]
- Kimple AJ, Torres AD, Yang RZ, et al. HPV-associated head and neck cancer: molecular and nano-scale markers for prognosis and therapeutic stratification. Sensors (Basel) 2012;12:5159-69. [PubMed]
- Poljak M, Cerar A, Seme K. Human papillomavirus infection in esophageal carcinomas: a study of 121 lesions using multiple broad-spectrum polymerase chain reactions and literature review. Hum Pathol 1998;29:266-71. [PubMed]
- Matsha T, Erasmus R, Kafuko AB, et al. Human papillomavirus associated with oesophageal cancer. J Clin Pathol 2002;55:587-90. [PubMed]
- Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, et al. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol 2014;32:1812-7. [PubMed]
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. [PubMed]
- Blitzer GC, Smith MA, Harris SL, et al. Review of the clinical and biologic aspects of human papillomavirus-positive squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 2014;88:761-70. [PubMed]
- Castillo A, Aguayo F, Koriyama C, et al. Human papillomavirus in esophageal squamous cell carcinoma in Colombia and Chile. World J Gastroenterol 2006;12:6188-92. [PubMed]
- Dreilich M, Bergqvist M, Moberg M, et al. High-risk human papilloma virus (HPV) and survival in patients with esophageal carcinoma: a pilot study. BMC Cancer 2006;6:94. [PubMed]
- Li T, Lu ZM, Chen KN, et al. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis 2001;22:929-34. [PubMed]
- Pantelis A, Pantelis D, Ruemmele P, et al. p53 Codon 72 polymorphism, loss of heterozygosity and high-risk human papillomavirus infection in a low-incidence German esophageal squamous cell carcinoma patient cohort. Oncol Rep 2007;17:1243-8. [PubMed]
- Teng H, Li X, Liu X, et al. The absence of human papillomavirus in esophageal squamous cell carcinoma in East China. Int J Clin Exp Pathol 2014;7:4184-93. [PubMed]
- Koshiol J, Wei WQ, Kreimer AR, et al. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer 2010;127:93-100. [PubMed]
- Löfdahl HE, Du J, Näsman A, et al. Prevalence of human papillomavirus (HPV) in oesophageal squamous cell carcinoma in relation to anatomical site of the tumour. PLoS One 2012;7:e46538. [PubMed]
- Noori S, Monabati A, Ghaderi A. The prevalence of human papilloma virus in esophageal squamous cell carcinoma. Iran J Med Sci 2012;37:126-33. [PubMed]
- Nitta Y, Tanaka H, Masuda Y, et al. The quality of DNA recovered from the archival tissues of atomic bomb survivors is good enough for the single nucleotide polymorphism analysis in spite of the decade-long preservation in formalin. J Radiat Res 2002;43:65-75. [PubMed]
- Khleif SN, DeGregori J, Yee CL, et al. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci U S A 1996;93:4350-4. [PubMed]
- Lakshmi S, Rema P, Somanathan T. p16ink4a is a surrogate marker for high-risk and malignant cervical lesions in the presence of human papillomavirus. Pathobiology 2009;76:141-8. [PubMed]
- Ishikawa M, Fujii T, Saito M, et al. Overexpression of p16 INK4a as an indicator for human papillomavirus oncogenic activity in cervical squamous neoplasia. Int J Gynecol Cancer 2006;16:347-53. [PubMed]
- Shah NG, Trivedi TI, Tankshali RA, et al. Prognostic significance of molecular markers in oral squamous cell carcinoma: a multivariate analysis. Head Neck 2009;31:1544-56. [PubMed]
- Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006;24:736-47. [PubMed]
- Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer 2007;120:1731-8. [PubMed]
- Harris SL, Thorne LB, Seaman WT, et al. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck 2011;33:1622-7. [PubMed]
- Chau NG, Perez-Ordonez B, Zhang K, et al. The association between EGFR variant III, HPV, p16, c-MET, EGFR gene copy number and response to EGFR inhibitors in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck Oncol 2011;3:11. [PubMed]
- Shuyama K, Castillo A, Aguayo F, et al. Human papillomavirus in high- and low-risk areas of oesophageal squamous cell carcinoma in China. Br J Cancer 2007;96:1554-9. [PubMed]
- Antonsson A, Nancarrow DJ, Brown IS, et al. High-risk human papillomavirus in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2010;19:2080-7. [PubMed]
- Malik SM, Nevin DT, Cohen S, et al. Assessment of immunohistochemistry for p16INK4 and high-risk HPV DNA by in situ hybridization in esophageal squamous cell carcinoma. Int J Surg Pathol 2011;19:31-4. [PubMed]
- Herbster S, Ferraro CT, Koff NK, et al. HPV infection in Brazilian patients with esophageal squamous cell carcinoma: interpopulational differences, lack of correlation with surrogate markers and clinicopathological parameters. Cancer Lett 2012;326:52-8. [PubMed]
- Cao F, Han H, Zhang F, et al. HPV infection in esophageal squamous cell carcinoma and its relationship to the prognosis of patients in northern China. ScientificWorldJournal 2014;2014:804738.
- Kawakami H, Okamoto I, Terao K, et al. Human papillomavirus DNA and p16 expression in Japanese patients with oropharyngeal squamous cell carcinoma. Cancer Med 2013;2:933-41. [PubMed]
- Salam I, Hussain S, Mir MM, et al. Aberrant promoter methylation and reduced expression of p16 gene in esophageal squamous cell carcinoma from Kashmir valley: a high-risk area. Mol Cell Biochem 2009;332:51-8. [PubMed]
- Sitas F, Egger S, Urban MI, et al. InterSCOPE study: Associations between esophageal squamous cell carcinoma and human papillomavirus serological markers. J Natl Cancer Inst 2012;104:147-58. [PubMed]
- Meschede W, Zumbach K, Braspenning J, et al. Antibodies against early proteins of human papillomaviruses as diagnostic markers for invasive cervical cancer. J Clin Microbiol 1998;36:475-80. [PubMed]
- Heideman DA, Waterboer T, Pawlita M, et al. Human papillomavirus-16 is the predominant type etiologically involved in penile squamous cell carcinoma. J Clin Oncol 2007;25:4550-6. [PubMed]
- Herrero R, Castellsagué X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-83. [PubMed]
- Zumbach K, Hoffmann M, Kahn T, et al. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in patients with head-and-neck squamous-cell carcinoma. Int J Cancer 2000;85:815-8. [PubMed]
- Furihata M, Ohtsuki Y, Ogoshi S, et al. Prognostic significance of human papillomavirus genomes (type-16, -18) and aberrant expression of p53 protein in human esophageal cancer. Int J Cancer 1993;54:226-30. [PubMed]
- Hippeläinen M, Eskelinen M, Lipponen P, et al. Mitotic activity index, volume corrected mitotic index and human papilloma-virus suggestive morphology are not prognostic factors in carcinoma of the oesophagus. Anticancer Res 1993;13:677-81. [PubMed]
- Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000;92:709-20. [PubMed]
- Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261-9. [PubMed]
- Qi Z, Jiang Q, Yang J, et al. Human papillomavirus (HPV) infection and the risk of esophageal squamous cell carcinoma. Dis Esophagus 2013;26:61-7. [PubMed]
- de Villiers EM, Gunst K, Stein H, et al. Esophageal squamous cell cancer in patients with head and neck cancer: Prevalence of human papillomavirus DNA sequences. Int J Cancer 2004;109:253-8. [PubMed]
- Ludmir EB, Palta M, Zhang X, et al. Incidence and prognostic impact of high-risk HPV tumor infection in cervical esophageal carcinoma. J Gastrointest Oncol 2014;5:401-7. [PubMed]


